| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 1,512 | 1,666 | 07.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| MOLECURE Aktie jetzt für 0€ handeln | |||||
| 28.03.25 | Strategic update on the progress and development of Molecure's key clinical projects in 2024 | 335 | GlobeNewswire (Europe) | Molecure achieved key milestones in the development of its innovative therapies in 2024 and plans to accelerate research and development in its most advanced clinical programs: OATD-01 and OATD-02.... ► Artikel lesen |
1 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| CO.DON | 0,017 | -21,43 % | EQS-PVR: CO.DON AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Stimmrechtsmitteilung: CO.DON AG
CO.DON AG: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung
07.01.2026 / 12:38 CET/CEST
Veröffentlichung... ► Artikel lesen | |
| SANGAMO THERAPEUTICS | 0,339 | +1,14 % | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| IMMATICS | 7,620 | -6,22 % | Immatics Presents IMA203CD8 PRAME Cell Therapy Data from Ongoing Dose Escalation and Shows Promising Initial Anti-tumor Activity in PRAME-Positive Tumors at ESMO-IO 2025 Congress | IMA203CD8 is a second-generation PRAME cell therapy with enhanced pharmacology in ongoing Phase 1a dose escalationManageable tolerability across all dose levelsEncouraging early clinical anti-tumor... ► Artikel lesen | |
| ALDEYRA | 4,700 | +0,38 % | Aldeyra Therapeutics, Inc.: Aldeyra Therapeutics to Participate in the Jefferies Global Healthcare Conference in London | Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced... ► Artikel lesen | |
| MEDIFAST | 9,172 | -4,46 % | Medifast: OPTAVIA Premieres Second Docuseries Episode "Health by Design: Spain" Exploring Metabolic Health and Longevity | Thousands of Independent Coaches Gather Nationwide to Discuss Solutions to America's Metabolic Health Crisis Medifast (NYSE: MED), the health and wellness company known for its science-backed... ► Artikel lesen | |
| PHARMING | 1,390 | +1,46 % | Pharming Group N.V.: Pharming Group announces 2026 financial guidance and highlights rare disease pipeline at Investor Day | Highlights advancing clinical-stage pipeline, including two major value-creating programs for primary immunodeficiencies (PIDs) with immune dysregulation and mtDNA-driven mitochondrial diseaseIntroduces... ► Artikel lesen | |
| COHERUS ONCOLOGY | 1,747 | -3,29 % | Coherus Biosciences stock gains on JNJ clinical supply agreement | ||
| ORUKA THERAPEUTICS | 26,000 | -3,70 % | Oruka Therapeutics, Inc.: Oruka Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Update | ORKA-001 Phase 1 results presented at EADV show potential for once-per-year dosing, higher efficacy and off-treatment remission Over $500M cash and equivalents provides runway over one year past three... ► Artikel lesen | |
| INNOVIVA | 18,300 | -1,08 % | FDA Approves Innoviva's NUZOLVENCE, First-in-Class Oral Treatment For Gonorrhea | ||
| ARMATA PHARMACEUTICALS | 6,700 | +0,75 % | Armata Pharmaceuticals, Inc.: Armata Pharmaceuticals Announces End-of-Phase 2 Meeting with FDA and Plans to Advance AP-SA02 to a Phase 3 Superiority Study in Complicated Staphylococcus aureus Bacteremia | FDA agreed that data from the Phase 2a diSArm study support advancement of AP-SA02 to a Phase 3study
First bacteriophage company to advance a clinical candidate... ► Artikel lesen | |
| SYNDAX PHARMACEUTICALS | 17,500 | -1,69 % | Syndax Pharmaceuticals, Inc.: Syndax Pharmaceuticals Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | ||
| DBV TECHNOLOGIES | 3,520 | -3,43 % | DBV Technologies S.A.: Information Regarding the Total Number of Voting Rights and Total Number of Shares of the Company as of January 31, 2026 | Information Regarding the Total Number of Voting Rights and Total Number of Shares of the Company as of January 31, 2026
(Article 223-16 of the General Regulations of the Autorité des Marchés Financiers)
Market... ► Artikel lesen | |
| BRAINSTORM CELL THERAPEUTICS | 0,673 | -100,00 % | BrainStorm Cell Therapeutics Inc.: BrainStorm Cell Therapeutics Announces Third Quarter 2025 Financial Results and Provides Corporate Update | NEW YORK, Nov. 14, 2025 /PRNewswire/ -- BrainStorm Cell Therapeutics Inc. (OTCQB: BCLI), a leading developer of adult stem cell therapeutics for neurodegenerative... ► Artikel lesen | |
| LIQUIDIA | 35,660 | -1,98 % | Liquidia Technologies, Inc.: Liquidia Corporation Announces Preliminary Full-Year 2025 YUTREPIA Net Sales and Corporate Update | Estimated YUTREPIA net product sales of approximately $90.1 million in the fourth quarter and $148.3 million for full-year 2025Received more than 2,800 unique patient prescriptions since launch in... ► Artikel lesen | |
| SIGA TECHNOLOGIES | 6,700 | +2,92 % | SIGA Technologies - Fundamentals intact amid several moving parts | Q325 was a quieter quarter for SIGA following strong Q2 sales, although this was not unexpected given its contract-driven model and inherent revenue lumpiness. No product sales were recorded, with total... ► Artikel lesen |
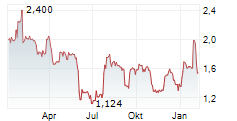
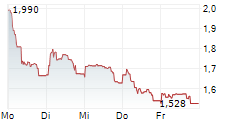
Das Unternehmen MOLECURE SA kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 1,528 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MOLECURE SA finden Sie auf Wallstreet Online.