- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Protagenic Therapeutics beruft William Nichols Jr. zum President | 2 | Investing.com Deutsch | ||
| Di | Protagenic Therapeutics, Inc.\new - 8-K, Current Report | - | SEC Filings | ||
| 24.02. | Protagenic Therapeutics, Inc.\new - 8-K, Current Report | - | SEC Filings | ||
| 18.02. | Protagenic Therapeutics, Inc.\new - NT 10-Q, Notification of inability to timely file Form 10-Q or 10-QSB | 2 | SEC Filings | ||
| 23.01. | NSE - Protagenic Therapeutics, Inc.\new - 25, Notification of the removal from listing and registration of matured, redeemed or retired securities | 2 | SEC Filings | ||
| 05.01. | Protagenic Therapeutics wechselt nach NASDAQ-Delisting in den OTC-Handel | 6 | Investing.com Deutsch | ||
| 05.01. | Protagenic Therapeutics, Inc.\new - 8-K, Current Report | 1 | SEC Filings | ||
| 09.12.25 | Protagenic Therapeutics, Inc.: Results from Phase 1 Multiple-Dose Study of PT00114 | 364 | ACCESS Newswire | Findings support advancement into Phase 2 to explore a first-in-class pathway aimed at stress-related neuropsychiatric conditions NEW YORK CITY, NEW YORK / ACCESS Newswire / December 9, 2025 / Protagenic... ► Artikel lesen | |
| PROTAGENIC THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 27.11.25 | Protagenic Therapeutics receives Nasdaq non-compliance letter | 5 | Seeking Alpha | ||
| 27.11.25 | Protagenic Therapeutics Announces Receipt of Nasdaq Non-Compliance Notice | 467 | ACCESS Newswire | NEW YORK, NY / ACCESS Newswire / November 27, 2025 / Protagenic Therapeutics, Inc. (Nasdaq:PTIX) (the "Company") today announced that it has received a notification letter from the Nasdaq Listing Qualifications... ► Artikel lesen | |
| 26.11.25 | Protagenic Therapeutics, Inc.\new - 10-Q, Quarterly Report | - | SEC Filings | ||
| 26.11.25 | Protagenic Therapeutics, Inc.\new - 8-K, Current Report | - | SEC Filings | ||
| 14.11.25 | Protagenic Therapeutics, Inc.\new - NT 10-Q, Notification of inability to timely file Form 10-Q or 10-QSB | - | SEC Filings | ||
| 13.11.25 | Protagenic-Aktie steigt nach Abschluss der Phase-1-Dosierung | 7 | Investing.com Deutsch | ||
| 13.11.25 | Protagenic schließt Dosierungsphase in Phase-1-Studie für Medikament gegen Stresserkrankungen ab | 2 | Investing.com Deutsch | ||
| 13.11.25 | Protagenic completes dosing in phase 1 study of stress disorder drug | 2 | Investing.com | ||
| 13.11.25 | Protagenic Therapeutics, Inc.: Protagenic Therapeutics Announces Completion of Enrollment and Dosing in Phase 1 MAD Study | 254 | ACCESS Newswire | NEW YORK CITY, NY / ACCESS Newswire / November 13, 2025 / Protagenic Therapeutics Inc. (NASDAQ:PTIX), a biopharmaceutical company developing novel therapeutics for stress-related neuropsychiatric and... ► Artikel lesen | |
| 31.10.25 | Protagenic Therapeutics reicht Klage zur Rückabwicklung der Übernahme von Phytanix Bio ein | 2 | Investing.com Deutsch | ||
| 31.10.25 | Protagenic Therapeutics, Inc.\new - 8-K, Current Report | 2 | SEC Filings | ||
| 22.08.25 | Protagenic Therapeutics, Inc.: Protagenic Therapeutics Provides Update on Form 10-Q Filing Timeline and Nasdaq Compliance | 535 | ACCESS Newswire | Delay Attributable to Merger-Related Financial Consolidation NEW YORK, NY / ACCESS Newswire / August 22, 2025 / Protagenic Therapeutics, Inc. (Nasdaq:PTIX), a biopharmaceutical company developing novel... ► Artikel lesen |
1 2 Weiter >>
26 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| MEDIGENE | 0,035 | -10,20 % | Medigene: Zurückziehung - 18.11.2025 | ||
| VALNEVA | 4,448 | +0,41 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| BIOFRONTERA | 2,770 | +2,97 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| 4SC | 0,100 | -23,37 % | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 16.12.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 16.12.2025Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 16.12.2025ISIN NameDE000A3E5C40 4SC... ► Artikel lesen | |
| PALATIN TECHNOLOGIES | 20,010 | 0,00 % | Palatin Technologies, Inc.: Palatin Reports Second Quarter Fiscal Year 2026 Financial Results and Provides Corporate Update | Advancing differentiated MC4R-based obesity programs into clinical development
Melanocortin-based obesity therapies targeting rare MC4R pathway disorders with... ► Artikel lesen | |
| INOVIO PHARMACEUTICALS | 1,450 | 0,00 % | INOVIO Pharmaceuticals, Inc.: FDA Accepts for Review INOVIO's BLA for INO-3107 for the Treatment of Adults with Recurrent Respiratory Papillomatosis (RRP) | PLYMOUTH MEETING, Pa., Dec. 29, 2025 /PRNewswire/ -- INOVIO (NASDAQ: INO), a biotechnology company focused on developing and commercializing DNA medicines to help... ► Artikel lesen | |
| SANGAMO THERAPEUTICS | 0,351 | +4,49 % | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| VIKING THERAPEUTICS | 27,780 | +1,04 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| SIRONA BIOCHEM | 0,037 | -100,00 % | Sirona Biochem Corp: Sirona Biochem downgraded to NEX | ||
| EDITAS MEDICINE | 1,736 | -1,42 % | Editas Medicine: Strategischer Fokus auf In-vivo-CRISPR-Therapie | ||
| VAXART | 0,560 | 0,00 % | Vaxart, Inc.: Vaxart Provides Business Update and Reports Third Quarter 2025 Financial Results | Entered into an exclusive license agreement with Dynavax for the Company's COVID-19 oral pill vaccine candidate for potential cumulative proceeds of up to $700 million plus royalties Completed enrollment... ► Artikel lesen | |
| IMMUNITYBIO | 8,566 | -0,53 % | ImmunityBio Q4 2026 Earnings Preview | ||
| MICROBOT MEDICAL | 2,094 | +1,95 % | Microbot Medical Inc.: Microbot Medical Confirms Continued Operational and Commercial Stability Amid Current Geopolitical Event | HINGHAM, Mass., March 03, 2026 (GLOBE NEWSWIRE) -- Microbot Medical Inc. (Nasdaq: MBOT), developer and distributor of the innovative LIBERTY Endovascular Robotic System, announced today that the Company... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,465 | -4,55 % | Recursion Pharmaceuticals: Recursion Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Delivered first clinical validation of the Recursion full stack AI Operating System in FAP, demonstrating translation from AI-driven biological insight to meaningful patient outcomes; multiple clinical... ► Artikel lesen | |
| AIM IMMUNOTECH | 0,073 | -100,00 % | AIM ImmunoTech Inc.: AIM ImmunoTech Signs Agreement for Planning of a Proposed Phase 3 Clinical Trial of Ampligen in the Treatment of Late-Stage Pancreatic Cancer | OCALA, Fla., March 02, 2026 (GLOBE NEWSWIRE) -- AIM ImmunoTech Inc. (NYSE American: AIM) ("AIM" or the "Company") today announced an agreement with the PPD clinical research business of Thermo Fisher... ► Artikel lesen |
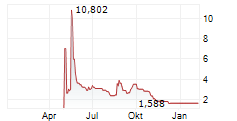
PROTAGENIC THERAPEUTICS INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,042 (-2,56 %) liegt der Kurs bei 1,588 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PROTAGENIC THERAPEUTICS INC finden Sie auf Wallstreet Online.