- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 02.02. | Advicenne: Official Reimbursement of Sibnayal for the Treatment of dRTA in France | 1.263 | Business Wire | Regulatory News:
Advicenne (Euronext Growth Paris FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from... ► Artikel lesen | |
| 27.01. | Advicenne Receives Marketing Authorization and Reimbursement for Sibnayal in UAE | 314 | Business Wire | Regulatory News:
Advicenne (Euronext Growth Paris FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from... ► Artikel lesen | |
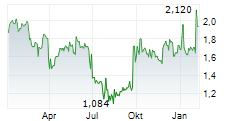
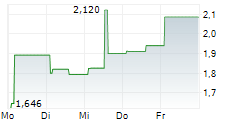
| 22.01. | Advicenne: 2025 Full Year Sales | 348 | Business Wire | 2025 sales totaled €5.73m, up 18.4%
Sibnayal sales rose 36.6% to €3.01m
2025 Royalties are estimated at €1m, double the amount for 2024
End-markets estimated sales of Sibnayal... ► Artikel lesen | |
| 20.01. | Advicenne: 2026 Financial Calendar | 306 | Business Wire | Regulatory News:
Advicenne(Euronext Growth Paris ALDVI FR0013296746), a specialty pharmaceutical company dedicated to the development and commercialization of innovative treatments for those suffering... ► Artikel lesen | |
| 19.01. | Advicenne's Sibnayal NDA For DRTA Tratement Accepted For Review By FDA | 2 | RTTNews | ||
| 19.01. | Advicenne: FDA Has Accepted for Review the Marketing Authorization Application for Sibnayal in the United States for the Treatment of dRTA | 262 | Business Wire | The US regulatory agency has set the target date for the final approval decision, known as the PDUFA date, as of September 3rd, 2026.
Regulatory News:
Advicenne (Euronext Growth Paris FR0013296746... ► Artikel lesen | |
| 12.01. | Advicenne Has Successfully Renewed the Marketing Authorization of Sibnayal in European Union | 240 | Business Wire | Regulatory News:
Advicenne (Euronext Growth Paris FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from... ► Artikel lesen | |
| ADVICENNE Aktie jetzt für 0€ handeln | |||||
| 04.11.25 | Advicenne Has Submitted to US FDA the Registration Application for Sibnayal in dRTA Treatment | 436 | Business Wire | Regulatory News:
Advicenne (Euronext Growth Paris FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from... ► Artikel lesen | |
| 04.11.25 | ADVICENNE: Advicenne has submitted to the US FDA the registration application (NDA) for Sibnayal in dRTA treatment | 4 | Euronext | ||
| 07.10.25 | ADVICENNE: Advicenne reports First Half financial results as of June 30, 2025, and updates on its activities | 5 | Euronext | ||
| 19.09.25 | Advicenne Reports First Half Financial Results as of June 30, 2025, and Updates on Its Activities | 490 | Business Wire | Sibnayal® end-market sales (Europe and Middle East) up 103% to €5.5 million
H1 products sales and royalties totaled €3.1 million, up 20%
Financial visibility extended to the fourth... ► Artikel lesen | |
| 26.08.25 | Advicenne Announces the Publication in Orphanet Journal of Rare Diseases of the Long-term European Study Results of dRTA Treatment Using ADV7103 | 364 | Business Wire | This extensive clinical dataset serves ADV7103 registration dossier in the United States of America, expected to be submitted in Q4 2025
Regulatory News:
Advicenne (Euronext Growth Paris... ► Artikel lesen | |
| 28.07.25 | Advicenne Receives Marketing Authorization and Reimbursement for Sibnayal in Saudi Arabia | 502 | Business Wire | Regulatory News:
Advicenne (Euronext Growth® FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from rare... ► Artikel lesen | |
| 25.07.25 | Advicenne Reports its H1 2025 Sales | 437 | Business Wire | Regulatory News:
Advicenne (Euronext Growth FR0013296746 ALDVI), a pharmaceutical company specializing in the development and marketing of innovative treatments for people suffering from rare kidney... ► Artikel lesen | |
| 21.07.25 | Advicenne Raises 2.6 Million Euros in Its Capital Increase and Extends Its Financial Visibility | 861 | Business Wire | Subscription of €2.6 million, representing 100% of the initial amount Financial horizon extended to the end of Q3 2026 thanks to concomitant financial restructuring Bpifrance now... ► Artikel lesen | |
| 02.07.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 02.07.2025 | 444 | Xetra Newsboard | Das Instrument 3MM FR0013296746 ADVICENNE (PROM.) EO-,20 EQUITY wird ex Kapitalmassnahme gehandelt am 02.07.2025 The instrument 3MM FR0013296746 ADVICENNE (PROM.) EO-,20 EQUITY is traded ex capital... ► Artikel lesen | |
| 30.06.25 | Advicenne Confirms the Upcoming Filing for Registration of ADV7103 in the USA and is Securing Its Financing Through Loan Restructuring and the Launch of a Rights Issue. | 470 | Business Wire | Submission of the US marketing authorization application for ADV7103 planned in Q4 2025, following positive feedback from the FDA on the additional data submitted. Repayment of tranches... ► Artikel lesen | |
| 29.04.25 | Advicenne 2024 Universal Registration Document Made Available | 370 | Business Wire | Regulatory News:
Advicenne (Euronext Growth Paris ALDVI FR0013296746), a specialty pharmaceutical company dedicated to the development and commercialization of innovative treatments for those suffering... ► Artikel lesen | |
| 27.03.25 | Advicenne: Fiscal Year 2024 Marked by the Satisfactory Commercial Performance of Sibnayal in Europe and Development in the United States | 669 | Business Wire | Total product sales of Advicenne up 9.4% to €4.9m
Operating cash consumption below €1M by 2025 and significant improvement in cash flow
Cash position of €3.2 million on December... ► Artikel lesen |
19 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| PFIZER | 23,350 | +0,19 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| ABBVIE | 199,80 | -0,35 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| HAEMATO | 10,700 | +7,00 % | M1 Kliniken schließt Verkauf von Haemato Pharm ab | Die M1 Kliniken AG hat den Verkauf der Haemato Pharm GmbH über ihre Mehrheitsbeteiligung Haemato AG an die Phoenix Group mit Wirkung zum Ablauf des 31. Januar 2026 abgeschlossen. Sämtliche Vollzugsbedingungen... ► Artikel lesen | |
| HALEON | 4,636 | -0,62 % | Haleon FY25 Profit Climbs, Revenues Down; Sees Growth In FY26, Mid-term; Plans GBP 500 Mln Buyback | LONDON (dpa-AFX) - Haleon plc (HLN, HKN.L), a consumer healthcare company, reported Wednesday higher profit in fiscal 2025, despite weak revenues. Further, the company projects growth in adjusted... ► Artikel lesen | |
| ACHIEVE LIFE SCIENCES | 3,825 | -3,16 % | Achieve Life Sciences: Entscheidet der 20. Juni über Leben und Tod? | Seit unseres Trading-Tipps im Juli letzten Jahres konnte die Aktie von Achieve Life Sciences zwischenzeitlich über +150% zulegen, korrigierte in den letzten Wochen aber wieder um mehr als 20%. Das Unternehmen... ► Artikel lesen | |
| LIGAND PHARMACEUTICALS | 173,00 | 0,00 % | Expert Outlook: Ligand Pharmaceuticals Through The Eyes Of 5 Analysts | ||
| ROYALTY PHARMA | 40,150 | -1,13 % | Royalty Pharma plc: Royalty Pharma Appoints Kenneth Sun as Senior Vice President and Head of Asia to Expand Royalty Pharma's Global Platform | NEW YORK, March 02, 2026 (GLOBE NEWSWIRE) -- Royalty Pharma plc (Nasdaq: RPRX) today announced the appointment of Kenneth Sun as Senior Vice President and Head of Asia, effective May 2026. Ken will... ► Artikel lesen | |
| EISAI | 27,530 | +0,36 % | Eisai: WELIREG(R) (belzutifan) Plus LENVIMA(R) (lenvatinib) Reduced the Risk of Disease Progression or Death by 30% Compared to Cabozantinib in Certain Previously Treated Patients With Advanced Renal Cell Carcinoma (RCC) | This is the first positive Phase 3 trial of a multi-targeted tyrosine kinase inhibitor in combination with a HIF-2 alpha inhibitor, the first for patients with RCC whose disease progressed on or after... ► Artikel lesen | |
| DR REDDYS | 12,000 | -4,00 % | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | ||
| TG THERAPEUTICS | 24,845 | -1,31 % | TG Therapeutics, Inc.: TG Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Raises BRIUMVI Revenue Guidance | Fourth quarter and full year 2025 total revenue of $192.6 million and $616.3 million, including BRIUMVI U.S. net revenue of $182.7 million and $594.1 million, respectively Target guidance of approximately... ► Artikel lesen | |
| BETTERLIFE PHARMA | 0,029 | -14,93 % | BetterLife Pharma Inc.: BetterLife Pharma to Participate in YAFO Capital Access Asia Partnering Forum During JPM Week in San Francisco | Vancouver, British Columbia--(Newsfile Corp. - January 6, 2026) - BetterLife Pharma Inc. (CSE: BETR) (OTCQB: BETRF) (FSE: NPAU) ("BetterLife" or the "Company"), an emerging biopharmaceutical company... ► Artikel lesen | |
| ENTOURAGE HEALTH | 0,001 | 0,00 % | Ontario Securities Commission: OSC bans WeedMD ex Carr, again | ||
| WAVE LIFE SCIENCES | 11,700 | -0,85 % | Wave Life Sciences Ltd.: Wave Life Sciences Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | On track for INLIGHT clinical data update in 1Q 2026; fat loss similar to GLP-1 observed at three months and expected to continue over time and with higher doses of WVE-007 (INHBE GalNAc-siRNA), while... ► Artikel lesen | |
| KYOWA KIRIN | 15,200 | 0,00 % | Grunenthal Group: Grünenthal takes full ownership of Grünenthal Meds, the joint venture established with Kyowa Kirin for its established medicines brands | Grünenthal acquired Kyowa Kirin International's 49% stake in 'Grünenthal Meds', a joint venture formed in 2023 to market the established medicines portfolio from Kyowa Kirin International.Through... ► Artikel lesen | |
| IGC PHARMA | 0,242 | +1,68 % | IGC Pharma, Inc.: IGC Pharma Files Utility Patent Applications Covering AI-Based Data Harmonization Architecture Supporting Alzheimer's Research | POTOMAC, MD / ACCESS Newswire / February 26, 2026 / IGC Pharma, Inc. (NYSE American:IGC) ("IGC" or the "Company"), a clinical-stage biotechnology company developing therapeutics for Alzheimer's disease... ► Artikel lesen |
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ADVICENNE finden Sie auf Wallstreet Online.