| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 14,900 | 15,200 | 15:31 | |
| 14,800 | 15,100 | 15:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 12:06 | Grunenthal Group: Grünenthal takes full ownership of Grünenthal Meds, the joint venture established with Kyowa Kirin for its established medicines brands | 173 | PR Newswire | Grünenthal acquired Kyowa Kirin International's 49% stake in 'Grünenthal Meds', a joint venture formed in 2023 to market the established medicines portfolio from Kyowa Kirin International.Through... ► Artikel lesen | |
| 09.02. | Kyowa Kirin Expects Annual Revenue To Rise, Projects Higher Dividend | 288 | AFX News | TOKYO (dpa-AFX) - Kyowa Kirin Co., Ltd.(KYKOY, KY4.F, 4151.T), a Japanese pharmaceutical company, said on Monday that it expects an increase in revenue for fiscal 2026, helped by growth in its... ► Artikel lesen | |
| KYOWA KIRIN Aktie jetzt für 0€ handeln | |||||
| 09.02. | Kyowa Kirin Co., Ltd. Full Year Income Advances | 230 | AFX News | WASHINGTON (dpa-AFX) - Kyowa Kirin Co., Ltd. (KYKOY) released a profit for its full year that Increased, from the same period last yearThe company's earnings came in at JPY67.040 billion, or... ► Artikel lesen | |
| 06.02. | Zebra im Karneval und Fortuna-Stadion? Kyowa Kirin macht Seltene Erkrankungen sichtbar! | 331 | news aktuell | Düsseldorf (ots) - Millionen Menschen weltweit leben mit einer Seltenen Erkrankung, die aufgrund ihrer Seltenheit oft hohe zusätzliche Belastungen für die Betroffenen mit sich bringt. Zum Tag der Seltenen... ► Artikel lesen | |
| 02.02. | Amgen hands back eczema drug to Kyowa Kirin despite positive late-stage data | 15 | Pharmaceutical Technology | ||
| 31.01. | Kyowa Kirin erlangt Kontrolle über Entwicklungs- und Kommerzialisierungsprogramm für Rocatinlimab wieder, zeigt starkes Engagement im Bereich des ungedeckten medizinischen Bedarfs bei atopischer Dermatitis | 1.668 | GlobeNewswire (Deutschland) | Kyowa Kirin bestätigt Engagement für die Entwicklung von Rocatinlimab als lebensveränderndes differenziertes Feld mit bedeutendem Marktpotenzial.Die neuartige Ausrichtung von Rocatinlimab als in der... ► Artikel lesen | |
| 30.01. | Amgen ends collaboration agreement with Kyowa Kirin for eczema asset | 6 | Seeking Alpha | ||
| 30.01. | Kyowa Kirin Regains Global Rights To Atopic Dermatitis Drug After Amgen Collaboration Ends | 2 | Benzinga.com | ||
| 30.01. | Amgen, Kyowa Kirin End Collaboration On Atopic Dermatitis Drug Rocatinlimab | 712 | AFX News | THOUSAND OAKS (dpa-AFX) - Kyowa Kirin Co., Ltd. (KYKOY) said on Friday it has ended its development and commercialization partnership with Amgen for rocatinlimab, with the Japanese drugmaker... ► Artikel lesen | |
| 30.01. | Kyowa Kirin to Regain Control of Rocatinlimab Development and Commercialization Program, Demonstrating Strong Commitment to Address High Unmet Medical Need in Atopic Dermatitis | 417 | GlobeNewswire (Europe) | Kyowa Kirin affirms commitment to developing rocatinlimab as a life-changing differentiated asset with significant market potential.Rocatinlimab's novel approach as an investigational T-cell rebalancing... ► Artikel lesen | |
| 29.12.25 | Dividendenbekanntmachungen (29.12.2025) | 13.471 | wallstreetONLINE (Dividenden) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) ADWAYS INC JP3121970002 6,35 JPY 0,0344 EUR AGC INC JP3112000009 105 JPY 0,5701 EUR AMERICAN TOWER CORPORATION US03027X1000 1... ► Artikel lesen | |
| 23.12.25 | Biocon arm secures global rights for Hulio from Fujifilm Kyowa Kirin Biologics Co | 5 | capitalmarket.com | ||
| 11.12.25 | Kyowa Kirin meldet geplante Ernennung von Abdul Mullick zum President und Chief Executive Officer - bisheriger CEO Masashi Miyamoto bleibt Chairman | 488 | Business Wire | Wechsel in der Unternehmensführung werden nach Abschluss der Hauptversammlung im März 2026 wirksam Bekanntgabe folgt auf ein Jahr mit dualem CEO-/COO-Modell und bedeutet Rückkehr zur Führungsstruktur... ► Artikel lesen | |
| 11.12.25 | Kyowa Kirin announces management changes | 5 | Seeking Alpha | ||
| 11.12.25 | Kyowa Kirin names Abdul Mullick as next CEO effective March 2026 | 5 | Investing.com | ||
| 11.12.25 | Führungswechsel bei Kyowa Kirin: Abdul Mullick wird 2026 neuer CEO | 6 | Investing.com Deutsch | ||
| 11.12.25 | Kyowa Kirin Announces Proposed Appointment of Abdul Mullick to President and Chief Executive Officer, While Former CEO Masashi Miyamoto to Remain Chairman | 1.345 | Business Wire | Leadership Changes to Take Effect Following Conclusion of Ordinary General Meeting of Shareholders in March 2026 Announcement Follows Year of Dual CEO COO Model and Returns Company to Single... ► Artikel lesen | |
| 08.12.25 | Kura Oncology, Kyowa Kirin reveal Komsifti combo data for AML | 3 | Seeking Alpha | ||
| 08.12.25 | Kura Oncology, Inc.: Kura Oncology and Kyowa Kirin Report Combination Data for KOMZIFTI (Ziftomenib) with Venetoclax and Azacitidine in Newly Diagnosed and Relapsed/Refractory AML | 244 | GlobeNewswire (Europe) | - 86% (32/37) CRc and 73% (27/37) CR in newly diagnosed NPM1-m AML, with 68% (17/25) of CRc responders achieving molecular MRD negativity by central NGS - Median duration of complete response and... ► Artikel lesen | |
| 02.12.25 | Kura Oncology, Inc.: First U.S. Commercial Sale of KOMZIFTI Triggers $135 Million Milestone Payment to Kura Oncology Under Collaboration and License Agreement with Kyowa Kirin | 235 | GlobeNewswire (Europe) | SAN DIEGO, Dec. 02, 2025 (GLOBE NEWSWIRE) -- Kura Oncology, Inc. (Nasdaq: KURA, "Kura"), a biopharmaceutical company committed to realizing the promise of precision medicines for the treatment of... ► Artikel lesen |
1 2 3 Weiter >>
55 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,775 | -2,56 % | DAX schwächer, Gold, Silber stark: Zollsorgen, Hella, Siemens, Bayer, Rheinmetall, Hochtief ... | Der DAX hat sich am Freitag freundlich ins Wochenende verabschiedet. Er stieg am Ende 0,9 Prozent auf 25.260,69 Zähler. Der Start in die neue Woche sieht allerdings deutlich schwächer aus. Zollunsicherheit... ► Artikel lesen | |
| NOVO NORDISK | 31,430 | -0,77 % | Novo Nordisk: Nächster Nackenschlag | Im aufstrebenden Adipositas-Markt droht der dänische Biopharma-Riese Novo Nordisk weiter hinter den US-Wettbewerber Eli Lilly zurückzufallen. Neue Daten zum Hoffnungsträger CagriSema sorgen erneut für... ► Artikel lesen | |
| PFIZER | 23,505 | +0,49 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,26 | +0,08 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 125,75 | +0,08 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 126,24 | +0,13 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,165 | -3,51 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 82,03 | +0,35 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,000 | +0,77 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,60 | 0,00 % | AbbVie Announces Positive Topline Results from Phase 3 AFFIRM Study Evaluating SKYRIZI (Risankizumab) Subcutaneous Induction in Patients with Crohn's Disease | In the Phase 3 AFFIRM study in adults with moderately to severely active Crohn's disease, risankizumab (SKYRIZI®) achieved superiority for the co-primary and ranked... ► Artikel lesen | |
| ROCHE | 396,45 | -1,77 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,931 | -1,17 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 897,40 | +0,73 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| MERCK & CO | 104,60 | -0,19 % | US-Konzern Merck & Co will Pharmasparte aufspalten | RAHWAY (dpa-AFX) - Der US-Konzern Merck & Co spaltet seine Pharmasparte auf. Künftig soll das Geschäft mit Krebsmedikamenten als eigener Bereich geführt werden, teilte das Unternehmen am Montag mit.... ► Artikel lesen |
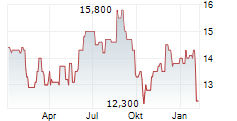
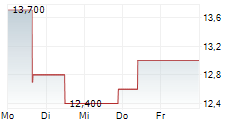
Das Unternehmen KYOWA KIRIN CO LTD kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,300 (-1,94 %) liegt der Kurs bei 15,200 Euro. Aus der Ausschüttung der letzten 12 Monate von insgesamt 0,34 Euro errechnet sich eine Dividendendrendite von +2,24 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu KYOWA KIRIN CO LTD finden Sie auf Wallstreet Online.