| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
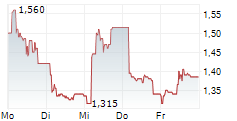
| 1,315 | 1,380 | 02.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 26.01. | Aelis Farma Announces Its 2026 Financial Calendar | 205 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS, PEA-PME eligible), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral... ► Artikel lesen | |
| 12.01. | Aelis Farma Receives a Positive Opinion From EMA Pediatric Committee on the Pediatric Investigation Plan for AEF0217 in Down Syndrome | 254 | Business Wire | The EMA's Pediatric Committee (PDCO) has delivered a favorable consensus opinion on the Pediatric Investigational Plan (PIP) of Aelis Farma's first-in-class drug candidate AEF0217 for the treatment... ► Artikel lesen | |
| 02.12.25 | Aelis Farma Obtains Regulatory Approval of the Phase 2B Trial With AEF0217 in People With Down Syndrome | 391 | Business Wire | AEF0217 is a first-in-class CB1 receptor Signalling Specific inhibitor (CB1-SSi) developed as a potential first pharmacological treatment for impairments in adaptive behaviours and cognition... ► Artikel lesen | |
| 25.09.25 | Aelis Farma: Availability of the 2025 Half-Year Financial Report | 344 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specialising in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 22.09.25 | Aelis Farma Reports 2025 Half-Year Financial Results and Presents Progress and Development Outlook | 515 | Business Wire | The first half of 2025 was marked by: The publication of the final analysis of the pioneering Phase 2b clinical trial in cannabis use disorders (CUD) with the CB1-SSi AEF0117, which further... ► Artikel lesen | |
| 24.06.25 | Aelis Farma: Availability of the Description of the Share Buyback Program | 399 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| AELIS FARMA Aktie jetzt für 0€ handeln | |||||
| 27.05.25 | Results of Aelis Farma Combined General Meeting of May 27, 2025 | 431 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 06.05.25 | Aelis Farma: Combined General Meeting of May 27, 2025: Availability of Preparatory Documents and Participation and Voting Procedures | 439 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 29.04.25 | Aelis Farma: Availability of the 2024 Universal Registration Document | 353 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 01.04.25 | Aelis Farma Reports Its 2024 Annual Financial Results and Confirms Its 2025 Outlook | 552 | Business Wire | 2024 has been marked by several significant events: The achievement of key milestones for the Company's two drug candidates:
AEF0117: announcement of the results of the Phase 2b clinical... ► Artikel lesen | |
| 26.03.25 | Aelis Farma Announces the Final Analysis of the Landmark Phase 2B Clinical Trial in Cannabis Use Disorder (CUD) with the CB1-SSi AEF0117 | 566 | Business Wire | The purpose of this pioneering Phase 2B trial was to show that AEF0117 reduces cannabis use and to determine the endpoints and optimal dosage to be used in future studies. AEF0117 is the first... ► Artikel lesen |
11 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| VIR BIOTECHNOLOGY | 9,340 | 0,00 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| ARCELLX | 114,05 | +0,22 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 90,10 | 0,00 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| BEAM THERAPEUTICS | 28,690 | +0,74 % | Beam Therapeutics Reports Fourth Quarter and Year-End 2025 Financial Results and Announces New Liver-Targeted Genetic Disease Program in Phenylketonuria (PKU) | New Program Designed as Platform-based Approach for Direct Correction of Mutations Causing PKU; Investigational New Drug (IND) Filing for BEAM-304 Anticipated in 2026 Updated Phase 1/2 Data and Next... ► Artikel lesen | |
| QIAGEN | 41,295 | -0,23 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,420 | -0,98 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| ARCUTIS BIOTHERAPEUTICS | 24,760 | -8,25 % | Arcutis Biotherapeutics, Inc.: Arcutis Announces Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Q4 2025 net product revenue for ZORYVE (roflumilast) was $127.5 million, an 84% increase compared to Q4 2024, and a 29% increase compared to Q3 2025Full year 2025 net product revenue for ZORYVE was... ► Artikel lesen | |
| ERASCA | 14,530 | +6,45 % | Erasca, Inc.: Erasca Announces Issuance of a U.S. Patent Covering Pan-KRAS Inhibitor ERAS-4001 | The issued patent provides intellectual property protection for ERAS-4001 and related compositions until at least 2043 Expands Erasca's diversified IP portfolio for RAS-driven cancers Initial Phase... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,630 | 0,00 % | Recursion Pharmaceuticals: Recursion Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Delivered first clinical validation of the Recursion full stack AI Operating System in FAP, demonstrating translation from AI-driven biological insight to meaningful patient outcomes; multiple clinical... ► Artikel lesen | |
| ADMA BIOLOGICS | 16,580 | 0,00 % | ADMA Biologics Announces $200 Mln Capital Return Plan | ||
| TREVI THERAPEUTICS | 12,570 | +5,54 % | Trevi Therapeutics, Inc.: Trevi Therapeutics Announces Appointment of David Hastings as Chief Financial Officer | Experienced biotech CFO to lead financial strategy and contribute to the Company's next stage of growth
NEW HAVEN, Conn., Dec. 4, 2025 /PRNewswire/ -- Trevi Therapeutics... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 70,92 | +1,26 % | RBC Capital lowers Apogee Therapeutics stock price target on dosing outlook | ||
| BIONTECH | 92,30 | -1,07 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 330,19 | -1,95 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| TYRA BIOSCIENCES | 32,520 | -2,63 % | Tyra Biosciences GAAP EPS of -$0.57 misses by $0.04 |
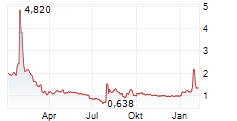
AELIS FARMA SAS gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 1,320 Euro und damit -2,94 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AELIS FARMA SAS finden Sie auf Wallstreet Online.