| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
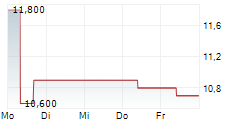
| 11,200 | 11,400 | 13:04 | |
| 11,200 | 11,600 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 03.02. | AKESO (09926): VOLUNTARY ANNOUNCEMENT - AKESO GRANTED EXCLUSIVE COMMERCIALIZATION RIGHTS FOR EBRONUCIMAB INJECTION TO JUMPCAN PHARMACEUTICAL | 4 | HKEx | ||
| 26.01. | AKESO (09926): CONTINUING CONNECTED TRANSACTION FURTHER INFORMATION IN RELATION TO THE CONTRACT MANUFACTURING ARRANGEMENT | 5 | HKEx | ||
| 07.01. | Akeso Secures NMPA Label Update For Ivonescimab With Dual Survival Benefits In NSCLC | 4 | RTTNews | ||
| 31.12.25 | AKESO (09926): CONTINUING CONNECTED TRANSACTIONS PURSUANT TO RULE 14A.60 OF THE LISTING RULES | 2 | HKEx | ||
| 12.12.25 | Akeso, Inc.: Cadonilimab (PD-1/CTLA-4) Receives FDA Clearance for Global Phase III First-Line Gastric Cancer Trial Versus Nivolumab | 267 | PR Newswire | HONG KONG, Dec. 11, 2025 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso" or the "Company") announced FDA approval to initiate COMPASSION-37/AK104-311 trial, a global... ► Artikel lesen | |
| AKESO Aktie jetzt für 0€ handeln | |||||
| 11.12.25 | Akeso, Inc.: Updated Efficacy Data of Ivonescimab Combined with Chemotherapy as First-Line Treatment for TNBC Presented at ESMO IO 2025 | 143 | PR Newswire | HONG KONG, Dec. 10, 2025 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso" or the "Company") announced the presentation of a Phase II clinical study that included... ► Artikel lesen | |
| 17.11.25 | Akeso, Inc.: Akeso's Bispecific Antibody Targeting Aß and BBB-Expressed Receptor Approved for Alzheimer's Disease Clinical Trials in China | 248 | PR Newswire | HONG KONG, Nov. 16, 2025 /PRNewswire/ -- Akeso, Inc. (HKEX: 9926.HK) announced that its AK152, a novel bispecific antibody targeting both amyloid-beta (Aß) and... ► Artikel lesen | |
| 10.11.25 | Akeso, Inc.: Akeso Announces First Patient Dosed in Phase I Trial of Personalized mRNA Vaccine AK154 as Monotherapy or in Combination with Cadonilimab or Ivonescimab for Adjuvant Treatment of Pancreatic Cancer | 160 | PR Newswire | HONG KONG, Nov. 10, 2025 /PRNewswire/ -- Akeso, Inc. (HKEX: 9926. HK) is pleased to announce that the first patient has been dosed in a Phase I clinical trial evaluating... ► Artikel lesen | |
| 07.11.25 | Akeso, Inc.: Ivonescimab HARMONi-A Study Final OS Analysis Results Presented at SITC 2025 with OS HR=0.74 | 392 | PR Newswire | The HARMONi-A study is the first Phase III trial of an immunotherapy for EGFR-TKI-resistant, EGFR-mutated non-squamous NSCLC to show statistically significant... ► Artikel lesen | |
| 20.10.25 | AKESO (09926): VOLUNTARY ANNOUNCEMENT - HARMONI-6 RESULTS PUBLISHED IN THE LANCET & 2025 ESMO MPFS 11.14M VS 6.9M (HR=0.60, P50.0001) FOR IVONESCIMAB ... | 5 | HKEx | ||
| 16.10.25 | Akeso, Inc.: HARMONi-6 Phase III Study of Ivonescimab Accepted by The Lancet and Selected for ESMO 2025 LBA Presentation | 286 | PR Newswire | HONG KONG, Oct. 16, 2025 /PRNewswire/ -- Akeso Inc. (9926.HK) today announced that the groundbreaking results from the registrational Phase III AK112-306/HARMONi-6... ► Artikel lesen | |
| 30.09.25 | AKESO (09926): 2025 INTERIM REPORT | 5 | HKEx | ||
| 23.09.25 | Akeso, Inc.: Ivonescimab HARMONi-6 Results Selected for ESMO 2025 LBA; Final Phase III Results of Cadonilimab as First-Line Therapy for Advanced Gastric Cancer to Be Published as an Oral Presentation | 247 | PR Newswire | HONG KONG, Sept. 22, 2025 /PRNewswire/ -- Akeso Inc. (9926.HK) is excited to announce that the Late-Breaking Abstract (LBA) from the registrational Phase III... ► Artikel lesen | |
| 22.09.25 | Akeso, Inc.: Akeso Announces First Patient Dosed in Registrational Phase II Study of TIGIT/TGF-ß Bifunctional Antibody Fusion Protein AK130 Combined with Ivonescimab for Advanced Pancreatic Cancer | 176 | PR Newswire | HONG KONG, Sept. 22, 2025 /PRNewswire/ -- Akeso Inc. (9926.HK) has announced that the first patient has been dosed in its registrational Phase II study (AK130-202)... ► Artikel lesen | |
| 16.09.25 | Akeso's cancer therapy gets orphan status from US FDA | 18 | Investing.com | ||
| 16.09.25 | Akeso's Ligufalimab Receives FDA Orphan Drug Designation For Acute Myeloid Leukemia | - | RTTNews | ||
| 16.09.25 | Akeso, Inc.: Akeso's Ligufalimab (CD47 mAb) Receives FDA Orphan Drug Designation for Acute Myeloid Leukemia (AML) | 222 | PR Newswire | HONG KONG, Sept. 15, 2025 /PRNewswire/ -- Akeso Inc. (9926.HK) today announced that its proprietary next-generation humanized IgG4 monoclonal antibody targeting... ► Artikel lesen | |
| 15.09.25 | Akeso, Inc.: Akeso Announces First Patient Dose in Global Registrational Trial of Cadonilimab (PD-1/CTLA-4) for PD-1 Treatment-Resistant Hepatocellular Carcinoma | 173 | PR Newswire | HONG KONG, Sept. 15, 2025 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso" or the "Company") announced that the first patient has been dosed in its global, multicenter... ► Artikel lesen | |
| 10.09.25 | Akeso, Inc.: Cadonilimab(PD-1/CTLA-4) plus Pulocimab (VEGFR-2) Combination Therapy Shows Promising Results in IO-Resistant Non-Small Cell Lung Cancer in Oral Presentation at the 2025 WCLC | 264 | PR Newswire | HONG KONG, Sept. 9, 2025 /PRNewswire/ -- Akeso Inc. (9926.HK) announced that data from a Phase Ib/II clinical study evaluating the combination of cadonilimab... ► Artikel lesen | |
| 04.09.25 | AKESO (09926): NEXT DAY DISCLOSURE RETURN | 2 | HKEx |
1 2 3 Weiter >>
42 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen |
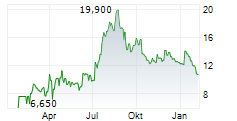
AKESO INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 11,400 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AKESO INC finden Sie auf Wallstreet Online.