| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
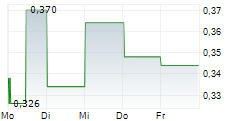
| 0,324 | 0,376 | 28.02. | |
| 0,326 | 0,376 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| ANTENGENE Aktie jetzt für 0€ handeln | |||||
| Mi | Junshi Biosciences Announces Strategic Collaboration with Antengene to Evaluate Combination Therapy with JS207 (PD-1/VEGF BsAb) and ATG-037 (Oral CD73 Inhibitor) | 390 | GlobeNewswire (Europe) | SHANGHAI, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Shanghai Junshi Biosciences Co., Ltd (Junshi Biosciences, HKEX: 1877; SSE: 688180), a leading innovation-driven biopharmaceutical company dedicated to the... ► Artikel lesen | |
| Mi | Antengene Corporation Limited: Antengene Announces Clinical Collaboration with Junshi Biosciences to Explore the Synergistic Potential of ATG-037 (Oral CD73 Inhibitor) In Combination with JS207 (PD-1/VEGF BsAb) | 586 | PR Newswire | SHANGHAI and HONG KONG, Feb. 24, 2026 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global... ► Artikel lesen | |
| 17.12.25 | Antengene Secures Malaysian Approval For XPOVIO In Relapsed/Refractory DLBCL | 1 | RTTNews | ||
| 16.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT XPOVIO APPROVED IN MALAYSIA FOR A NEW INDICATION IN DIFFUSE LARGE B-CELL LYMPHOMA | 2 | HKEx | ||
| 12.12.25 | ANTENGENE-B (06996): NOMINATION AND CORPORATE GOVERNANCE COMMITTEE - TERMS OF REFERENCE | 5 | HKEx | ||
| 03.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT XPOVIO APPROVED IN HONG KONG FOR TWO ADDITIONAL INDICATIONS IN MULTIPLE MYELOMA AND DIFFUSE LARGE B-CELL LYMPHOMA | 1 | HKEx | ||
| 02.12.25 | Antengene: NMPA Approves IND Application For Phase Ib/II CLINCH-2 Study With ATG-022 | - | RTTNews | ||
| 02.12.25 | Antengene Corporation Limited: Antengene Announces IND Approval in China for Phase Ib/II Study of ATG-022 (CLDN18.2 ADC) in Combination with KEYTRUDA (Pembrolizumab) ± Chemotherapy | 168 | PR Newswire | SHANGHAI and HONG KONG, Dec. 2, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global biotech... ► Artikel lesen | |
| 02.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT IND APPROVAL IN CHINA FOR PHASE IB/II STUDY OF ATG-022 IN COMBINATION WITH KEYTRUDA (PEMBROLIZUMAB) CHEMOTHERAPY | 2 | HKEx | ||
| 20.10.25 | Antengene Corporation Limited: Antengene Presents Latest ATG-022 Clinical Data at ESMO 2025 Demonstrating Efficacy Across All CLDN18.2 Expression Levels and Exceptional Tolerability | 529 | PR Newswire | SHANGHAI and HONG KONG, Oct. 20, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced the latest results from the ongoing Phase I/II CLINCH... ► Artikel lesen | |
| 15.10.25 | JPMorgan nimmt Antengene-Aktie mit "Overweight" in die Bewertung auf | 2 | Investing.com Deutsch | ||
| 15.10.25 | JPMorgan initiates Antengene stock coverage with Overweight rating | 1 | Investing.com | ||
| 29.09.25 | ANTENGENE-B (06996): 2025 INTERIM REPORT | 1 | HKEx | ||
| 19.09.25 | Antengene Corporation Limited: Antengene to Present Latest Preclinical Results from ATG-201 (CD19xCD3 TCE) at ACR 2025 | 378 | PR Newswire | SHANGHAI and HONG KONG, Sept. 19, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced that it will release the latest preclinical data of ATG-201... ► Artikel lesen | |
| 10.09.25 | ANTENGENE-B (06996): NEXT DAY DISCLOSURE RETURN | - | HKEx | ||
| 25.08.25 | Antengene Corporation Limited: Antengene Announces 2025 Interim Results with Encouraging Clinical Data and Progress in TCE Platform | 431 | PR Newswire | SHANGHAI and HONG KONG, Aug. 25, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced its interim results for the period ending June 30, 2025, along... ► Artikel lesen | |
| 22.08.25 | Antengene Corporation Limited: Antengene Announces 2025 Interim Financial Results Highlighting Encouraging Data from Mid/Late-Stage Clinical Programs and Its Innovative TCE Technology Platform | 210 | PR Newswire | The Phase I/II CLINCH study of ATG-022 (CLDN18.2 antibody-drug conjugate) demonstrated promising results, showing robust clinical efficacy and a favorable safety... ► Artikel lesen | |
| 19.08.25 | Antengene Corporation Limited: Antengene's ATG-022 (CLDN18.2 ADC) Granted Breakthrough Therapy Designation for the Treatment of Gastric/Gastroesophageal Junction Adenocarcinoma | 354 | PR Newswire | SHANGHAI and HONG KONG, Aug. 19, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global biotech company dedicated to... ► Artikel lesen | |
| 20.05.25 | Antengene Corporation Limited: Antengene Enters into a Global Clinical Collaboration with MSD to Evaluate ATG-022 (CLDN18.2 ADC) In Combination with KEYTRUDA (pembrolizumab) | 330 | PR Newswire | - ATG-022 is Antengene's CLDN18.2 antibody-drug conjugate; KEYTRUDA® (pembrolizumab) is MSD's anti-PD-1 therapy.
SHANGHAI and HONG KONG, May 20, 2025 /PRNewswire/... ► Artikel lesen | |
| 21.03.25 | Antengene Corporation Limited: Antengene Announces 2024 Full-Year Financial Results, Proprietary Programs Advancing to Pivotal Trials with Accelerating Multi-market Revenue Ramp Up | 272 | PR Newswire | SHANGHAI and HONG KONG, March 21, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced its full-year results for the... ► Artikel lesen |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| ILLUMINA | 113,72 | -0,09 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen |
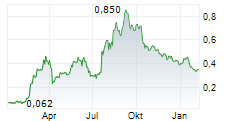
ANTENGENE CORP LTD gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 0,334 Euro und damit +0,60 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ANTENGENE CORP LTD finden Sie auf Wallstreet Online.