| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
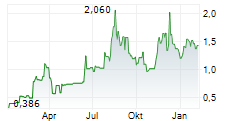
| 1,670 | 1,710 | 27.02. | |
| 1,650 | 1,700 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.02. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS SELECTS ORAL AMYLIN RECEPTOR PEPTIDE AGONIST, ASC36, FOR CLINICAL DEVELOPMENT | 2 | HKEx | ||
| ASCLETIS PHARMA Aktie jetzt für 0€ handeln | |||||
| 10.02. | ASCLETIS-B (01672): NEXT DAY DISCLOSURE RETURN | 4 | HKEx | ||
| 10.02. | ASCLETIS-B (01672): COMPLETION OF PLACING OF NEW SHARES UNDER THE GENERAL MANDATE | 1 | HKEx | ||
| 06.02. | Ascletis Pharma Inc.: GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital in China's Core Innovative Drug Assets | 376 | JCN Newswire | HONG KONG, Feb 6, 2026 - (ACN Newswire) - According to disclosure of interests filed with the Hong Kong Stock Exchange (HKEX), the Government of Singapore Investment Corporation (GIC) has acquired an... ► Artikel lesen | |
| 06.02. | Ascletis Pharma Inc.: GIC Private Limited initiated a stake in Ascletis Pharma (01672) by 64,128,000 shares at a price of HKD 12.18 per share | 314 | JCN Newswire | HONG KONG, Feb 6, 2026 - (ACN Newswire) - According to the disclosure of interests information released by the Hong Kong Stock Exchange, on 5 February, GIC Private Limited made its first equity investment... ► Artikel lesen | |
| 05.02. | Ascletis Pharma Inc.: GIC Private Limited initiated a stake in Ascletis Pharma (01672) by 64,128,000 shares at a price of HKD 12.18 per share | 181 | JCN Newswire | HONG KONG, Feb 5, 2026 - (ACN Newswire) - According to the disclosure of interests information released by the Hong Kong Stock Exchange, GIC Private Limited made its equity investment in Ascletis Pharma... ► Artikel lesen | |
| 05.02. | Sagimet announces positive results for Ascletis' denifanstat in moderate to severe acne | 8 | PMLiVE | ||
| 03.02. | ASCLETIS-B (01672): PLACING OF NEW SHARES UNDER THE GENERAL MANDATE | 5 | HKEx | ||
| 02.02. | Ascletis reports positive long-term safety data for acne drug denifanstat | 4 | Investing.com | ||
| 02.02. | Sagimet Biosciences Inc.: Sagimet Announces Positive 52-Week Data from License Partner Ascletis' Open-Label Phase 3 Clinical Trial Evaluating the Long-Term Safety of ASC40 (Denifanstat) Tablets in Patients with Moderate to Severe Acne | 275 | GlobeNewswire (Europe) | SAN MATEO, Calif., Feb. 02, 2026 (GLOBE NEWSWIRE) -- Sagimet Biosciences Inc. (Nasdaq: SGMT), a clinical-stage biopharmaceutical company developing novel therapeutics targeting dysfunctional metabolic... ► Artikel lesen | |
| 29.01. | Ascletis Pharma Inc.: Ascletis Announces Positive Topline Results from Its Phase III Open-Label Study of Denifanstat (ASC40), a First-in-Class, Once-Daily Oral FASN Inhibitor for Acne | 255 | PR Newswire | - Denifanstat (ASC40), a once-daily oral fatty acid synthase (FASN) inhibitor, demonstrated favorable safety and tolerability in a Phase III open-label study
-... ► Artikel lesen | |
| 29.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES POSITIVE TOPLINE RESULTS FROM ITS PHASE III OPEN-LABEL STUDY OF DENIFANSTAT (ASC40), A ... | - | HKEx | ||
| 26.01. | Ascletis Pharma Inc.: Ascletis Announces First Participants Dosed in a 13-week U.S. Phase II Study with ASC30, an Oral Small Molecule GLP-1R Agonist for the Treatment of Diabetes | 148 | PR Newswire | - Topline data from the Phase II study for the treatment of diabetes are expected in the third quarter of 2026.
-ASC30 demonstrated placebo-adjusted weight loss... ► Artikel lesen | |
| 26.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES FIRST PARTICIPANTS DOSED IN A 13-WEEK U.S. PHASE II STUDY WITH ASC30, AN ORAL SMALL MOLECULE ... | 3 | HKEx | ||
| 20.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS SELECTS A NEXT-GENERATION ONCE-MONTHLY SUBCUTANEOUSLY ADMINISTERED GLP-1R/GIPR/GCGR TRIPLE PEPTIDE ... | - | HKEx | ||
| 20.01. | Ascletis Pharma Inc.: Ascletis Selects a Next-Generation Once-Monthly Subcutaneously Administered GLP-1R/GIPR/GCGR Triple Peptide Agonist, ASC37, for Clinical Development | 217 | PR Newswire | - In head-to-head non-human primate (NHP) studies, average observed half-life of ASC37 was approximately 17 days, 7-fold longer than retatrutide, which supports... ► Artikel lesen | |
| 05.01. | Ascletis Pharma Inc.: Ascletis Announces U.S. FDA IND Clearance for 13-Week Phase II Study of Its Oral Small Molecule GLP-1, ASC30, in Participants with Diabetes | 169 | PR Newswire | -The Phase II study for diabetes is a 13-week, randomized, double-blind, placebo-controlled and multi-center study to evaluate the efficacy, safety, and tolerability... ► Artikel lesen | |
| 05.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES U.S. FDA IND CLEARANCE FOR 13-WEEK PHASE II STUDY OF ITS ORAL SMALL MOLECULE GLP-1, ASC30, ... | 1 | HKEx | ||
| 29.12.25 | ASCLETIS-B (01672): NEXT DAY DISCLOSURE RETURN | 1 | HKEx | ||
| 19.12.25 | ASCLETIS-B (01672): NEXT DAY DISCLOSURE RETURN | - | HKEx |
1 2 3 4 Weiter >>
75 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology |
SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
Das Unternehmen ASCLETIS PHARMA INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 1,700 Euro und damit +1,19 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASCLETIS PHARMA INC finden Sie auf Wallstreet Online.