| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 1,136 | 1,226 | 13:03 | |
| 1,148 | 1,214 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.02. | What Pushed Entera Bio (ENTX) To Rally Nearly 16% After Hours | 2 | Benzinga.com | ||
| 11.02. | Entera Bio Announces Open Market Purchases of Company Stock by Board Members | 2 | GlobeNewswire (USA) | ||
| 09.02. | Entera Bio Appoints Former Pfizer Executive Geno J. Germano as Chairman of the Board Ahead of Key Milestones | 74 | GlobeNewswire (Europe) | TEL AVIV, Feb. 09, 2026 (GLOBE NEWSWIRE) -- Entera Bio Ltd. (NASDAQ: ENTX) ("Entera" or the "Company"), a leader in the development of oral peptide and protein replacement therapies, today announced... ► Artikel lesen | |
| 04.02. | OPKO und Entera Bio vertiefen Partnerschaft für orale PTH-Behandlung | 11 | Investing.com Deutsch | ||
| 04.02. | OPKO and Entera Bio expand collaboration to develop oral PTH treatment | 2 | Investing.com | ||
| 04.02. | OPKO Health, Inc.: OPKO Health and Entera Bio Expand Partnership to Advance First-in-Class Oral Long Acting PTH Tablet for Patients with Hypoparathyroidism | 297 | GlobeNewswire (Europe) | This is the third program that successfully combines Entera's oral peptide N-Tab- platform with OPKO's advanced protein chemistry capabilities The companies have accelerated this program and aim... ► Artikel lesen | |
| ENTERA BIO Aktie jetzt für 0€ handeln | |||||
| 04.02. | OPKO Health and Entera Bio Expand Partnership to Advance First-in-Class Oral Long Acting PTH Tablet for Patients with Hypoparathyroidism | 1 | GlobeNewswire (USA) | ||
| 04.02. | Entera Bio Ltd. - 8-K, Current Report | 2 | SEC Filings | ||
| 21.01. | Entera Bio Announces Upcoming Q1 2026 Corporate Priorities and Pipeline Outlook | 260 | GlobeNewswire (Europe) | Final EB613 Phase 3 Protocol Submission to FDA Planned for Q1 2026, Following December 19th 2025 FDA Ruling Next-Generation EB613 Phase 1 Bridging Study Progressing with Results Expected During Q1... ► Artikel lesen | |
| 24.12.25 | FDA qualifiziert BMD-Endpunkt: H.C. Wainwright bestätigt "Buy"-Rating für Entera Bio | 8 | Investing.com Deutsch | ||
| 24.12.25 | H.C. Wainwright reiterates Buy rating on Entera Bio stock as FDA qualifies BMD endpoint | 5 | Investing.com | ||
| 23.12.25 | Entera Bio Congratulates the FNIH-ASBMR-SABRE Team on FDA's Qualification of Total Hip BMD as Regulatory Endpoint: Huge Win for Osteoporosis Innovation | 4 | GlobeNewswire (USA) | ||
| 22.12.25 | Entera Bio's oral PTH analog shows sustained calcium response | 4 | Investing.com | ||
| 22.12.25 | Entera Bio Announces New Data Supporting Further Development of a Proprietary First-in-Class Oral, Long-Acting PTH Tablet for Patients with Hypoparathyroidism (EB612 Program) | 1 | GlobeNewswire (USA) | ||
| 14.11.25 | Entera Bio GAAP EPS of $0.07 | 1 | Seeking Alpha | ||
| 14.11.25 | Entera Bio Announces Third Quarter 2025 Financial Results and Business Updates | 558 | GlobeNewswire (Europe) | FDA Agreement on BMD as Primary Endpoint for EB613 Registrational, Phase 3 Study EB613 Phase 2 Data Demonstrating Consistent Efficacy across Younger Post-Menopausal Women with Osteoporosis and its... ► Artikel lesen | |
| 23.10.25 | Entera Bio reports positive data for oral osteoporosis treatment | 2 | Investing.com | ||
| 23.10.25 | Entera Bio Presents Positive New Clinical Data from EB613 Phase 2 Trial Demonstrating Significant Bone Density Improvements in Early Postmenopausal Women | 459 | GlobeNewswire (Europe) | Consistency of BMD gains presented at NAMS 2025 demonstrate EB613's efficacy in both young postmenopausal women and in women 10 years post-menopause Data further support EB613 potential as a first-in-class... ► Artikel lesen | |
| 16.10.25 | Entera Bio to Present New Clinical Data from Phase 2 Trial of EB613 at the 2025 North American Menopause Society (NAMS) Annual Meeting | 2 | GlobeNewswire (USA) | ||
| 25.09.25 | Entera Bio stock rating reiterated at Buy by H.C. Wainwright | 2 | Investing.com |
1 2 Weiter >>
29 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
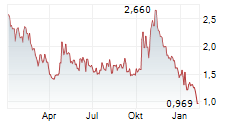
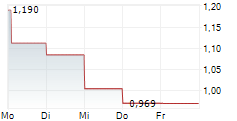
ENTERA BIO LTD gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,054 (-4,25 %) liegt der Kurs bei 1,216 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ENTERA BIO LTD finden Sie auf Wallstreet Online.