| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 3,700 | 3,820 | 11:10 | |
| 3,720 | 3,820 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - NEW DRUG APPLICATION OF DALMELITINIB MESYLATE TABLETS WAS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION | 2 | HKEx | ||
| HANSOH PHARMACEUTICAL Aktie jetzt für 0€ handeln | |||||
| 20.02. | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - AUMOLERTINIB MESYLATE TABLETS WAS APPROVED IN THE EU AS MONOTHERAPY | 6 | HKEx | ||
| 16.02. | Nomura Lifts HANSOH PHARMA's TP to HKD37.87 w/Higher Rev. & Earnings Forecast | 3 | AASTOCKS | ||
| 03.02. | HANSOH PHARMA (03692): COMPLETION OF THE ISSUE OF HK$4,680 MILLION ZERO COUPON CONVERTIBLE BONDS DUE 2033 | 1 | HKEx | ||
| 27.01. | Hansoh Pharmaceutical: Aktie stürzt nach Ankündigung von Wandelanleihe ab | 6 | Investing.com Deutsch | ||
| 26.01. | HANSOH PHARMA (03692): PROPOSED ISSUE OF HK$4,680 MILLION ZERO COUPON CONVERTIBLE BONDS DUE 2033 | 5 | HKEx | ||
| 08.01. | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - DRUG REGISTRATION APPROVAL OF THE FIFTH INDICATION OF AMEILE (AUMOLERTINIB MESYLATE TABLETS) WAS GRANTED ... | 3 | HKEx | ||
| 16.12.25 | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - LICENSE AGREEMENT WITH GLENMARK | 3 | HKEx | ||
| 08.12.25 | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - PROGRESS ON THE INCLUSION OF INNOVATIVE DRUGS IN THE 2025 NATIONAL REIMBURSEMENT DRUG LIST | 5 | HKEx | ||
| 23.10.25 | HANSOH PHARMA (03692): VOLUNTARY ANNOUNCEMENT - NEW DRUG APPLICATION OF HS-10365 CAPSULES WAS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION | 1 | HKEx | ||
| 17.10.25 | Licensing galore: Roche ties up with Hansoh on ADCs as Rani rounds out week with Chugai pact | 12 | FierceBiotech | ||
| 17.10.25 | Roche licenses cancer candidate from China's Hansoh Pharma | 12 | Seeking Alpha | ||
| 17.10.25 | HANSOH PHARMA (03692): INSIDE INFORMATION - LICENSE AGREEMENT WITH ROCHE | 1 | HKEx | ||
| 25.09.25 | JPMorgan initiates Hansoh Pharma stock with Overweight rating on innovation growth | 2 | Investing.com | ||
| 25.09.25 | JPMorgan initiates Hansoh Pharma stock with Overweight rating | 2 | Investing.com | ||
| 23.09.25 | HANSOH PHARMA (03692): INTERIM DIVIDEND FOR THE SIX MONTHS ENDED JUNE 30, 2025 (UPDATED) | 2 | HKEx | ||
| 23.09.25 | HANSOH PHARMA (03692): RESCHEDULE OF DATES FOR INTERIM DIVIDEND FOR THE SIX MONTHS ENDED JUNE 30, 2025 DUE TO WEATHER CONDITIONS | 1 | HKEx | ||
| 22.09.25 | Dividendenbekanntmachungen (22.09.2025) | 10.179 | wallstreetONLINE (Dividenden) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) AECON GROUP INC CA00762V1094 0,19 CAD 0,1173 EUR APT SATELLITE HOLDINGS LTD BMG0438M1064 0,025 HKD 0,0027 EUR ARROWMARK FINANCIAL... ► Artikel lesen | |
| 22.09.25 | HANSOH PHARMA (03692): NOTIFICATION LETTER TO NON-REGISTERED HOLDERS AND REQUEST FORM | 2 | HKEx | ||
| 22.09.25 | HANSOH PHARMA (03692): NOTIFICATION LETTER TO REGISTERED SHAREHOLDERS AND REPLY FORM | 2 | HKEx |
1 2 Weiter >>
29 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Bayer Aktie: Kursrally am absoluten Limit - droht jetzt der große Rücksetzer? | © Foto: sbDie Bayer-Aktie hat in kurzer Zeit eine beeindruckende Aufholjagd hingelegt. Vom Tief bei unter 20 Euro schoss der Kurs auf knapp 50 Euro - mehr als eine Verdopplung. Klingt gut, aber genau... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Novo Nordisk: Nächster Nackenschlag | Im aufstrebenden Adipositas-Markt droht der dänische Biopharma-Riese Novo Nordisk weiter hinter den US-Wettbewerber Eli Lilly zurückzufallen. Neue Daten zum Hoffnungsträger CagriSema sorgen erneut für... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,44 | +0,21 % | dpa-AFX-Überblick: UNTERNEHMEN - Die wichtigsten Meldungen vom Wochenende | GESAMT-ROUNDUP 2: Kommt eine Social-Media-Altersgrenze ab 14 Jahren? STUTTGART - Ein Mindestalter für soziale Medien wie Tiktok und Instagram zum Schutz von Kindern und Jugendlichen rückt immer näher.... ► Artikel lesen | |
| MERCK KGAA | 128,60 | +2,35 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,41 | -0,40 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,040 | +0,93 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| ROCHE | 402,50 | -0,27 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,950 | +0,85 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| ELI LILLY | 889,90 | -0,11 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| MERCK & CO | 104,60 | -0,19 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen | |
| ASTRAZENECA | 177,25 | +0,45 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen |
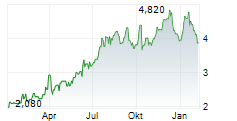
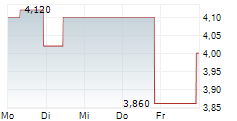
HANSOH PHARMACEUTICAL GROUP CO LTD gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von -0,080 (-2,12 %) liegt der Kurs bei 3,700 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HANSOH PHARMACEUTICAL GROUP CO LTD finden Sie auf Wallstreet Online.