- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Solid Biosciences Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| Fr | Solid Biosciences announces $240 million private placement | 1 | Seeking Alpha | ||
| Fr | Solid Biosciences sichert sich 240 Mio. US-Dollar durch Privatplatzierung | 3 | Investing.com Deutsch | ||
| Fr | Solid Biosciences secures $240M private placement funding | 1 | Investing.com | ||
| SOLID BIOSCIENCES Aktie jetzt für 0€ handeln | |||||
| Fr | Solid Biosciences: Aktie springt nach Privatplatzierung über 240 Mio. US-Dollar | 1 | Investing.com Deutsch | ||
| Fr | Solid Biosciences Inc.: Solid Biosciences Announces Oversubscribed $240 Million Private Placement | 2 | GlobeNewswire (USA) | ||
| Mo | Solid Biosciences Inc.: Solid Biosciences Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | 1 | GlobeNewswire (USA) | ||
| 10.02. | Citizens reiterates Market Outperform rating on Solid Biosciences stock | 1 | Investing.com | ||
| 09.02. | Solid Bio aligns with FDA on late-stage trial design for Duchenne drug | 2 | Seeking Alpha | ||
| 09.02. | Solid Biosciences advances Duchenne treatment with FDA alignment | 3 | Investing.com | ||
| 06.02. | Solid Biosciences Flies Ahead of Summit | 2 | Baystreet.ca | ||
| 06.02. | Solid Biosciences Inc.: Solid Biosciences to Present at the Guggenheim Emerging Outlook: Biotech Summit 2026 | 2 | GlobeNewswire (USA) | ||
| 15.01. | JPM26: Solid Biosciences banks on its AAV capsid to catalyse gene therapy success | 3 | Pharmaceutical Technology | ||
| 14.01. | Solid Bio rises as Duchenne study gets fully enrolled | 3 | Seeking Alpha | ||
| 13.01. | Gentherapie-Entwickler Solid Biosciences meldet Fortschritte in klinischen Studien | 1 | Investing.com Deutsch | ||
| 13.01. | Solid Biosciences advances gene therapy trials for rare diseases | 1 | Investing.com | ||
| 13.01. | Solid Biosciences Inc.: Solid Biosciences Provides 2026 Outlook Underscoring Neuromuscular and Cardiac Pipeline Momentum and Expanded Access to Next-Generation Capsid AAV-SLB101 | 229 | GlobeNewswire (Europe) | - Duchenne: Dosed 33 participants in the Phase 1/2 INSPIRE DUCHENNE clinical trial as of January 9, 2026; SGT-003 continues to be generally well tolerated using a steroid-only prophylactic immunomodulation... ► Artikel lesen | |
| 12.01. | Solid Biosciences Inc.: Solid Biosciences Receives FDA Orphan Drug Designation for SGT-212 Dual-Route Gene Therapy for the Treatment of Friedreich's Ataxia | 578 | GlobeNewswire (Europe) | - SGT-212 has received FDA Fast Track, Rare Pediatric Disease and Orphan Drug designations - - Dosing of the first participant in the Phase 1b FALCON trial has been completed, with initial data expected... ► Artikel lesen | |
| 12.01. | Solid Biosciences startet klinische Studie zu Friedreich-Ataxie mit erster Patientendosierung | - | Investing.com Deutsch | ||
| 12.01. | Solid Biosciences doses first participant in Friedreich's ataxia trial | 2 | Investing.com |
1 2 3 Weiter >>
52 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 87,05 | -0,51 % | BioNTech-Konkurrent Moderna: Satter Kurssprung - jetzt noch einsteigen? | Die Aktie von Moderna hat zuletzt erneut kräftigen Rückenwind erhalten. Die Korrektur seit Ende Januar wurde damit nicht nur vollständig wieder ausgeglichen, das Papier konnte sogar auf ein neues Jahreshoch... ► Artikel lesen | |
| EVOTEC | 5,402 | -1,13 % | Shortseller-Positionen aktuell: Evotec, Gerresheimer, HelloFresh, Renk, Hypoport, Symrise, TUI | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| BB BIOTECH | 49,800 | -0,60 % | Christian Koch, Head BB Biotech: «Opportunitäten entstehen dort, wo Fortschritt und Bewertung noch nicht im Gleichgewicht sind» | ||
| QIAGEN | 39,095 | -3,59 % | Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 45,130 | -0,29 % | Moderna freut sich über eine Zulassungsempfehlung der EMA für einen Kombi-Impfstoff gegen Corona und Grippe | Bei Corona-Impfstoffen war der US-Konzern Moderna noch ein Nachzügler. Anders scheint es nun bei Kombi-Impfstoffen zu laufen, die gleichzeitig vor Corona und der Grippe schützen. Dafür gibt es bisher... ► Artikel lesen | |
| VALNEVA | 4,548 | -0,48 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 316,00 | -0,74 % | Leerink Partners senkt Amgen-Prognose für Q1 wegen Lagerbestandsabbau | ||
| EPIGENOMICS | 0,960 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,567 | -0,22 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 314,60 | +0,16 % | William Blair reiterates Outperform on Stryker stock after analyst briefing | ||
| BIOGEN | 157,65 | -1,00 % | Biogen Inc.: The New England Journal of Medicine Publishes First Data to Demonstrate the Potential for Disease Modification in Dravet Syndrome | -Results show that targeting the underlying cause of Dravet syndrome with zorevunersen may improve outcomes for people with this rare, devastating genetic neurodevelopmental disease-
-Data support... ► Artikel lesen | |
| BIOFRONTERA | 2,680 | +2,68 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 3,030 | -2,26 % | EQS-Adhoc: Heidelberg Pharma AG: Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von Soleus Capital bekannt | EQS-Ad-hoc: Heidelberg Pharma AG / Schlagwort(e): Bedeutende Verträge
Heidelberg Pharma und HealthCare Royalty geben Änderung der bestehenden Lizenzvereinbarung und Beteiligung von... ► Artikel lesen | |
| ILLUMINA | 107,60 | +0,19 % | Illumina (ILMN) Gaining from Stronger Than Expected Results | ||
| CRISPR THERAPEUTICS | 48,200 | -1,23 % | Forget CRISPR Therapeutics: This Gene-Editing Player Already Boasts the Profits It Dreams Of |
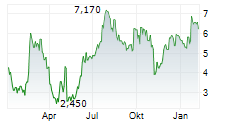
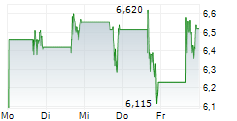
Das Unternehmen SOLID BIOSCIENCES INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 6,750 Euro und damit +20,43 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SOLID BIOSCIENCES INC finden Sie auf Wallstreet Online.