- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 18.02. | Aprea Therapeutics Advanced Cancer Trial Shows Promising Results, Stock Soars | 4 | Benzinga.com | ||
| 18.02. | Aprea meldet zweite partielle Remission in Studie zu Endometriumkarzinom | 2 | Investing.com Deutsch | ||
| 18.02. | Aprea Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 18.02. | Aprea reports second partial response in endometrial cancer trial | 1 | Investing.com | ||
| APREA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 13.02. | EQS Newswire: Aprea Therapeutics: Aprea Therapeutics Strengthens Global Patent Portfolio in DNA Damage Response (DDR) Cancer Therapeutics, Paving Way for Pipeline Growth | 363 | EQS Group (EN) | EQS Newswire / 13/02/2026 / 09:45 UTC+8
New patents granted in 2025 in Australia and Japan bolster global IP coverage for Aprea's WEE1 and ATR programs. Core patent families are expected to provide... ► Artikel lesen | |
| 12.02. | Aprea Therapeutics Strengthens Global Patent Portfolio in DNA Damage Response (DDR) Cancer Therapeutics, Paving Way for Pipeline Growth | 149 | GlobeNewswire (Europe) | New patents granted in 2025 in Australia and Japan bolster global IP coverage for Aprea's WEE1 and ATR programs. Core patent families are expected to provide exclusivity into 2045. Lead WEE1 inhibitor... ► Artikel lesen | |
| 12.02. | EQS Newswire: Aprea Therapeutics: Aprea Therapeutics Strengthens Global Patent Portfolio in DNA Damage Response (DDR) Cancer Therapeutics, Paving Way for Pipeline Growth | 375 | EQS Group (EN) | EQS Newswire / 12/02/2026 / 10:14 UTC+8
New patents granted in 2025 in Australia and Japan bolster global IP coverage for Aprea's WEE1 and ATR programs. Core patent families are expected to provide... ► Artikel lesen | |
| 04.02. | Aprea Therapeutics appoints Eugene Kennedy as chief medical advisor | 2 | Investing.com | ||
| 04.02. | Aprea Therapeutics Appoints Industry Veteran Eugene Kennedy, MD, as Chief Medical Advisor | 375 | GlobeNewswire (Europe) | DOYLESTOWN, Pa., Feb. 04, 2026 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) ("Aprea" or the "Company"), a clinical-stage biopharmaceutical company developing innovative therapies that... ► Artikel lesen | |
| 30.01. | Weekly Buzz: Intellia Gets FDA Nod For ATTRv-PN Trial; Aprea's APR-1051 Paces; CALC Halts KOURAGE | 735 | AFX News | SOUTH SAN FRANCISCO (dpa-AFX) - This week's biotech landscape features key FDA approvals, clinical holds and go-aheads, trial discontinuations, and clinical trial data readouts across key therapeutic... ► Artikel lesen | |
| 29.01. | Aprea meldet erstes partielles Ansprechen in Phase-1-Studie für Krebsmedikament | 8 | Investing.com Deutsch | ||
| 29.01. | Aprea Therapeutics prices $5.6M private placement | 7 | Seeking Alpha | ||
| 29.01. | Aprea Therapeutics raises $5.6 million in private placement | 6 | Investing.com | ||
| 29.01. | Aprea reports first partial response in Phase 1 cancer drug trial | 2 | Investing.com | ||
| 29.01. | Aprea Therapeutics Announces $5.6 Million Private Placement Priced At-The-Market Under Nasdaq Rules | 164 | GlobeNewswire (Europe) | DOYLESTOWN, Pa., Jan. 29, 2026 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) ("Aprea", or the "Company"), a clinical-stage biopharmaceutical company developing innovative treatments... ► Artikel lesen | |
| 29.01. | Aprea Therapeutics Announces Early Clinical Proof-Of-Concept in the Ongoing ACESOT-1051 Dose-Escalation Trial Evaluating WEE1 Inhibitor APR-1051, Including Partial Response Observed on First Scan | 144 | GlobeNewswire (Europe) | Approximately 50% reduction in target lesion and greater than 90% decrease in CA-125 observed in endometrial cancer patientThe unconfirmed partial response (uPR) that was observed in the first scan... ► Artikel lesen | |
| 23.01. | Aprea Therapeutics, Inc. - 8-K, Current Report | 4 | SEC Filings | ||
| 09.01. | Aprea Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 18.12.25 | Aprea Therapeutics reports progress in cancer drug development | 16 | Investing.com | ||
| 18.12.25 | Fortschritte bei Krebstherapie: Aprea Therapeutics meldet Studiendaten und sichert Liquidität | 11 | Investing.com Deutsch |
1 2 Weiter >>
38 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 49,400 | -3,89 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| VALNEVA | 4,402 | -6,93 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| BIOGEN | 163,00 | +0,40 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| ILLUMINA | 113,48 | -0,30 % | Evercore ISI reiterates Illumina stock rating on competitive positioning | ||
| ABIVAX | 100,80 | -1,95 % | 2 Reasons Abivax Stock Could 10X by 2036 | ||
| GINKGO BIOWORKS | 5,750 | +0,88 % | Ginkgo Bioworks Reports Fourth Quarter and Full Year 2025 Financial Results, Announces Focus on Autonomous Labs Offerings and Divestiture of its Non-Core Biosecurity Business | Ginkgo provides an update on its fourth quarter financial results
BOSTON, Feb. 26, 2026 /PRNewswire/ -- Ginkgo Bioworks Holdings, Inc. (NYSE: DNA, "Ginkgo") today... ► Artikel lesen | |
| ONCO-INNOVATIONS | 0,428 | +2,88 % | Onco-Innovations Limited: Onco-Innovations Announces Amendment to Private Placement Pricing | Not for distribution to United States wire services or for dissemination in the United States VANCOUVER, BC / ACCESS Newswire / February 12, 2026 / Onco-Innovations Limited (CBOE CA:ONCO)(Frankfurt:W1H... ► Artikel lesen | |
| QIAGEN | 41,960 | +1,38 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 62,98 | -2,63 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | -79,74 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | -2,26 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | +24,33 % | Cardio Diagnostics Holdings, Inc. - 8-K, Current Report | ||
| ADMA BIOLOGICS | 15,580 | +2,70 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance |
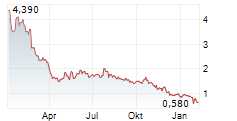
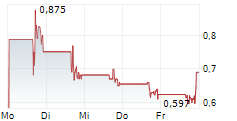
Das Unternehmen APREA THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 0,900 Euro und damit +2,27 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu APREA THERAPEUTICS INC finden Sie auf Wallstreet Online.