| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
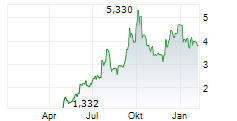
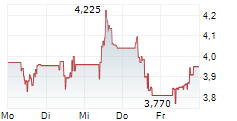
| 4,385 | 4,450 | 02.03. | |
| 4,395 | 4,435 | 02.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Idorsia überzeugt in Q4 2025 mit starkem Wachstum in Kernmärkten | 35 | Investing.com Deutsch | ||
| Do | Idorsia verdoppelt 2025 Umsatz und reduziert den Verlust | 439 | Moneycab | Allschwil - Das Biotechunternehmen Idorsia schaut auf ein bewegtes 2025 zurück. Dabei ist es dem Unternehmen gelungen, weiter zu wachsen. Der Umsatz kletterte 2025 auf 221 Millionen Franken von 113... ► Artikel lesen | |
| Do | Novartis und Idorsia: Das Pharma-Powerpaar von Basel | 41 | Schweizer Fernsehen | ||
| Do | Idorsia Pharmaceuticals Ltd: Idorsia reports strong 2025 results with QUVIVIQ sales more than doubling - further sales growth ahead with multiple pipeline catalysts in 2026 | 430 | GlobeNewswire (Europe) | Ad hoc announcement pursuant to Art. 53 LR
Idorsia delivers on its upgraded 2025 guidance, with strong QUVIVIQ sales growth, disciplined investment, and a significantly improved bottom line.Focused... ► Artikel lesen | |
| 12.02. | Idorsia Pharmaceuticals Ltd: Invitation to Idorsia's FY 2025 Financial Reporting webcast and conference call | 381 | GlobeNewswire (Europe) | Idorsia will publish its Full Year 2025 Financial Reporting on Thursday February 26, at 07:00 CET.
An investor webcast and conference call will be held to discuss the results on the same day.
Date:... ► Artikel lesen | |
| IDORSIA Aktie jetzt für 0€ handeln | |||||
| 06.02. | FDA gibt grünes Licht für Idorsias Fabry-Mittel | 69 | cash | ||
| 06.02. | Idorsia Pharmaceuticals Ltd: Clear route to registration positions lucerastat as the potential first oral therapy for all patients with Fabry disease | 685 | GlobeNewswire (Europe) | Allschwil, Switzerland - February 06, 2026Idorsia Ltd (SIX: IDIA) announces the design of its FDA-agreed Phase 3 registration program for lucerastat in Fabry disease. Building on the robust biomarker... ► Artikel lesen | |
| 30.01. | Idorsia Pharmaceuticals Ltd: Idorsia to present long-term lucerastat data and kidney biopsy results at WORLDSymposium | 438 | GlobeNewswire (Europe) | Data from up to 42 months of treatment reinforce lucerastat's potential as a first-in-class oral substrate reduction therapy addressing key unmet needs across the Fabry populationThe company is finalizing... ► Artikel lesen | |
| 28.01. | Idorsia Pharmaceuticals Ltd: Global expansion of Idorsia's QUVIVIQ continues with EMS partnership for Latin America | 832 | GlobeNewswire (Europe) | Ad hoc announcement pursuant to Art. 53 LR
Regulatory dossier to obtain marketing authorization for QUVIVIQ has been submitted to ANVISA in Brazil
Allschwil, Switzerland - January 28, 2026Idorsia... ► Artikel lesen | |
| 12.01. | Idorsia Advances Fabry Disease Program With Encouraging Phase 3 Results | 21 | RTTNews | ||
| 12.01. | Idorsia Pharmaceuticals Ltd: Nature Communications reports promising effect of Idorsia's lucerastat on kidney function in Fabry disease | 448 | GlobeNewswire (Europe) | In patients with impaired renal function or fast-deteriorating eGFR at baseline, lucerastat was associated with a marked attenuation of kidney function loss, suggesting a potential disease-modifying... ► Artikel lesen | |
| 12.01. | Idorsia Pharmaceuticals Ltd: Idorsia to present at J.P. Morgan 2026 Healthcare Conference | 547 | GlobeNewswire (Europe) | Allschwil, Switzerland - January 12, 2026Idorsia Ltd (SIX: IDIA) announces that Srishti Gupta, MD, Chief Executive Officer of Idorsia, will present at the 44th Annual J.P. Morgan Healthcare Conference... ► Artikel lesen | |
| 06.01. | Idorsia Pharmaceuticals Ltd: Idorsia initiates a proof-of-concept trial with its oral first-in-class selective CCR6 antagonist | 578 | GlobeNewswire (Europe) | The trial aims to establish clinical proof-of-concept in psoriasis and proof-of-mechanism for other CCR6- and Th17-associated autoimmune indications
Allschwil, Switzerland - January 6, 2026Idorsia... ► Artikel lesen | |
| 05.01. | Idorsia Pharmaceuticals Ltd: Idorsia's daridorexant in women during menopausal transition age with insomnia | 2.848 | GlobeNewswire (Europe) | New analysis of a Phase 3 study of daridorexant shows that 50 mg improved both sleep and daytime functioning compared to placebo in women during menopausal transition ageBrigham and Women's Hospital... ► Artikel lesen | |
| 05.01. | Idorsia Pharmaceuticals Ltd: Idorsia's JERAYGO (aprocitentan) approved in Canada for the treatment of resistant hypertension | 679 | GlobeNewswire (Europe) | Idorsia receives approval from Health Canada for JERAYGO (aprocitentan) as the first and only endothelin receptor antagonist (ERA) for the treatment of resistant hypertension.JERAYGO is a new oral antihypertensive... ► Artikel lesen | |
| 17.12.25 | Idorsia: Struktureller Neustart: Für die heimische Biotech-Schmiede beginnt ein neues Kapitel. ... | 127 | payoff.ch | ||
| 10.12.25 | Idorsia Highlights New PRECISION Study Analysis Showing Strong Results For Aprocitentan | 24 | RTTNews | ||
| 10.12.25 | Idorsia Pharmaceuticals Ltd: New Hypertension publication underscores aprocitentan's potential in managing hypertension patients with CKD | 568 | GlobeNewswire (Europe) | New analysis from landmark Phase 3 PRECISION trial published in Hypertension highlights the renal-protective benefits of aprocitentan in addition to the marked reduction in blood pressure in a high-risk... ► Artikel lesen | |
| 09.12.25 | Idorsia Pharmaceuticals Ltd: Idorsia's treatment for insomnia disorder wins the inaugural Prix Galien Bridges Award in the 'Best Biotechnology & Pharmaceutical Product' category | 621 | GlobeNewswire (Europe) | Idorsia's dual orexin receptor antagonist, the first and only drug of its kind approved in Europe for the treatment of insomnia disorder, has been awarded the inaugural Prix Galien Bridges Award in... ► Artikel lesen | |
| 01.12.25 | Kepler Cheuvreux stuft Idorsia nach Umschuldung auf "Kaufen" hoch | 102 | Investing.com Deutsch |
1 2 3 4 Weiter >>
70 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,30 | -1,07 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,686 | -2,94 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,60 | +0,39 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,036 | -10,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,295 | -0,23 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 45,140 | -0,45 % | Moderna Stock (MRNA): Is It a Buy Now? 7 Questions After the Crash | ||
| PAION | 0,070 | -22,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,742 | +0,25 % | Valneva SE - 6-K, Report of foreign issuer | ||
| AMGEN | 329,30 | +0,23 % | AAPL, AA, BKR, and AMGN are among the top stocks for momentum, says Oppenheimer | ||
| EPIGENOMICS | 1,020 | -2,86 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,765 | +2,18 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,90 | +0,88 % | $100 Invested In Stryker 15 Years Ago Would Be Worth This Much Today | ||
| BIOGEN | 161,20 | -0,71 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,560 | -4,48 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,870 | -5,90 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
IDORSIA AG gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +2,92 % bei einem Aktienkurs von 4,410 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IDORSIA AG finden Sie auf Wallstreet Online.