| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 6,940 | 6,960 | 11:48 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 03.02. | MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | 289 | Business Wire | Article 223-16 of the General Regulations of the Financial Markets Authority
(AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| 20.01. | MaaT Pharma startet Phase-2-Studie mit Mikrobiom-Therapie bei Lungenkrebs | 9 | Investing.com Deutsch | ||
| 20.01. | MaaT Pharma Announces First Patient Randomized in IMMUNOLIFE Phase 2 Study Sponsored by Gustave Roussy, To Explore the Role of the Gut Microbiome To Overcome ICI Resistance in Advanced NSCLC Patients with Antibiotic-Induced Dysbiosis | 476 | Business Wire | IMMUNOLIFE, a Phase 2 randomized exploratory study, will evaluate the potential of MaaT033 in combination with Cemiplimab versus Best Investigator Choice (i.e.: second line) in enhancing disease... ► Artikel lesen | |
| MAAT PHARMA Aktie jetzt für 0€ handeln | |||||
| 10.12.25 | Original-Research: MaaT Pharma SACA (von First Berlin Equity Research GmbH): BUY | 362 | EQS Group (EN) | Original-Research: MaaT Pharma SACA - from First Berlin Equity Research GmbH
10.12.2025 / 11:25 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer... ► Artikel lesen | |
| 08.12.25 | MaaT Pharma reports 54% one-year survival rate in pivotal GvHD trial | 4 | Investing.com | ||
| 08.12.25 | MaaT Pharma meldet 54 % Ein-Jahres-Überlebensrate in zulassungsrelevanter GvHD-Studie | 6 | Investing.com Deutsch | ||
| 08.12.25 | MaaT Pharma Presents Pivotal ARES Phase 3 Results for MaaT013 (Xervyteg) in Acute GvHD at ASH 2025 Annual Congress and Announces 54% 1-Year Overall Survival | 679 | Business Wire | Presentation included previously disclosed primary results from the pivotal ARES Phase 3 single-arm trial evaluating MaaT013 (Xervyteg) in treating refractory severe acute Graft-versus-Host Disease... ► Artikel lesen | |
| 19.11.25 | Original-Research: MaaT Pharma SACA (von First Berlin Equity Research GmbH): BUY | 324 | EQS Group (EN) | Original-Research: MaaT Pharma SACA - from First Berlin Equity Research GmbH
19.11.2025 / 11:11 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer... ► Artikel lesen | |
| 17.11.25 | XFRA 4RD: WIEDERAUFNAHME/RESUMPTION | 150 | Xetra Newsboard | FOLGENDE(S) INSTRUMENT(E) WIRD/ WERDEN WIEDER IN DEN HANDEL AUFGENOMMEN MIT FOLGENDEM TRADING SCHEDULE.THE FOLLOWING INSTRUMENT(S) IS/ARE RESUMED TRADING WITH FOLLOWING TRADING SCHEDULE:INSTRUMENT NAME... ► Artikel lesen | |
| 14.11.25 | MaaT Pharma Announces the Successful Completion of Its Global Offering of €9.1 Million | 457 | Business Wire | Regulatory News:
THIS ANNOUNCEMENT AND THE INFORMATION CONTAINED HEREIN IS RESTRICTED AND IS NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN, INTO... ► Artikel lesen | |
| 14.11.25 | XFRA 4RD: AUSSETZUNG/SUSPENSION | 292 | Xetra Newsboard | DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTILMAAT PHARMA S.A.... ► Artikel lesen | |
| 13.11.25 | MaaT Pharma Launches a Capital Increase of Approximately €9 Million | 611 | Business Wire | Launch of a Global Offering of new ordinary shares for approximately €9 million through a Private Placement aimed at qualified investors, and a PrimaryBid Offering aimed at retail investors via... ► Artikel lesen | |
| 05.11.25 | MaaT Pharma Presents Updated Preclinical Data at SITC Annual Meeting Demonstrating Immune Activation and Anti-Tumor Activity of MaaT034 | 399 | Business Wire | Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage biotechnology company and a leader in the development of Microbiome Ecosystem TherapiesTM (MET) dedicated to enhancing... ► Artikel lesen | |
| 04.11.25 | Original-Research: MaaT Pharma SACA (von First Berlin Equity Research GmbH): BUY | 293 | EQS Group (EN) | Original-Research: MaaT Pharma SACA - from First Berlin Equity Research GmbH
04.11.2025 / 11:37 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer... ► Artikel lesen | |
| 04.11.25 | MaaT Pharma Reports Financial Results for the Third Quarter 2025 and Provides Financing Update | 366 | Business Wire | The Company received an upfront payment of €10.5 million in July 2025 after the signature of an exclusive license and distribution agreement with Clinigen. Subject to approval and grant of a Marketing... ► Artikel lesen | |
| 03.11.25 | MaaT Pharma's Xervyteg shows 62% response rate in GI-aGvHD trial | 5 | Investing.com | ||
| 03.11.25 | MaaT Pharma: Xervyteg erzielt 62 % Ansprechrate in entscheidender Studie zu GI-aGvHD | 4 | Investing.com Deutsch | ||
| 03.11.25 | MaaT Pharma Announces Positive Phase 3 Results Evaluating Xervyteg (MaaT013) in Acute Graft-versus-Host Disease Selected for Oral Presentation at ASH Congress 2025 | 388 | Business Wire | Oral presentation at ASH 2025 to feature pivotal Phase 3 results of Xervyteg (MaaT013), including previously disclosed primary endpoint data (62% GI-ORR at Day 28) and new findings on secondary... ► Artikel lesen | |
| 07.10.25 | MaaT Pharma stock jumps 11% after DSMB clears Phase 2b trial to continue | 6 | Investing.com | ||
| 07.10.25 | MaaT Pharma's MaaT033 For Allo-HSCT Patients Clears Second DSMB Safety Review In Phase 2b Trial | 2 | RTTNews |
1 2 3 Weiter >>
43 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BB BIOTECH | 50,70 | -1,74 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| INTELLIA THERAPEUTICS | 12,340 | -6,69 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces FDA Lift of Clinical Hold on MAGNITUDE Phase 3 Clinical Trial in ATTR-CM | CAMBRIDGE, Mass., March 02, 2026 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (Nasdaq: NTLA), a leading biopharmaceutical company focused on revolutionizing medicine leveraging CRISPR gene editing... ► Artikel lesen | |
| KUROS BIOSCIENCES | 29,480 | -1,67 % | Kuros Biosciences AG: Kuros Biosciences to provide investor update at Octavian and Baader Bank Conferences | Kuros Biosciences to provide investor updateat Octavian and Baader Bank Conferences
Schlieren (Zürich), Switzerland, January 13, 2026 - Kuros Biosciences ("Kuros" or the "Company") a leader in innovative... ► Artikel lesen | |
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,413 | +1,64 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| MICROBOT MEDICAL | 2,000 | -5,93 % | H.C. Wainwright bestätigt Rating für Microbot Medical nach Adoption durch Klinik | ||
| ATAIBECKLEY | 3,100 | -4,32 % | XETR NEW INSTRUMENTS AVAILABLE ON XETRA - 25.02.2026 | Das Instrument B72 US04650F1012 ATAIBECKLEY INC. O.N. EQUITY hat seinen ersten Handelstag am 25.02.2026: CONTINUOUS TRADING WITH AUCTIONS, PAG NAM0, SettlCurr EUR, CCP Y
The instrument B72 US04650F1012... ► Artikel lesen | |
| BIOMERIEUX | 99,55 | -1,53 % | BioMerieux SA Profit Declines In Full Year | PARIS (dpa-AFX) - BioMerieux SA (EYWN.MU) reported earnings for full year that Drops, from the same period last yearThe company's bottom line totaled EUR397.5 million, or EUR3.34 per share.... ► Artikel lesen | |
| BIOXCEL THERAPEUTICS | 1,378 | -0,86 % | BioXcel Therapeutics Planning to Submit sNDA This Month Seeking FDA Approval for At-Home Use of IGALMI | NEW HAVEN, Conn., Jan. 07, 2026 (GLOBE NEWSWIRE) -- BioXcel Therapeutics, Inc. (Nasdaq: BTAI), a biopharmaceutical company built on artificial intelligence ("AI") to develop transformative medicines... ► Artikel lesen | |
| CYBIN | 6,200 | -4,62 % | Helus Pharma Appoints Former Pfizer Chief Medical Officer Dr. Freda Lewis-Hall to Board of Directors & Chair of the Scientific Advisory Committee | In this role, Dr. Lewis-Hall will guide clinical development strategy, regulatory engagement, and translational rigor across Helus' novel serotonergic agonist ("NSA") portfolio This news release constitutes... ► Artikel lesen | |
| BIO GREEN MED SOLUTION | 1,020 | 0,00 % | Bio Green Med Solution, Inc.: Bio Green Med Solution Reports Third Quarter Financial Results and Provides Business Update | KUALA LUMPUR, Malaysia, Nov. 13, 2025 (GLOBE NEWSWIRE) -- Bio Green Med Solution, Inc. (NASDAQ: BGMS, NASDAQ: BGMSP; "BGMS" or the "Company" (formerly Cyclacel Pharmaceuticals, Inc.)), a diversified... ► Artikel lesen | |
| PDS BIOTECHNOLOGY | 0,579 | -1,95 % | PDS Biotechnology Corporation: PDS Biotech Announces Adoption of Amended Protocol for Phase 3 VERSATILE-003 Trial Incorporating Progression Free Survival (PFS) as Primary Endpoint for Interim Analysis and Potential Accelerated Approval | PRINCETON, N.J., Feb. 20, 2026 (GLOBE NEWSWIRE) -- PDS Biotechnology Corporation (Nasdaq: PDSB) ("PDS Biotech" or the "Company"), a late-stage immunotherapy company focused on transforming how the... ► Artikel lesen | |
| ZYMEWORKS | 19,800 | 0,00 % | Zymeworks Secures $250 Mln Royalty Funding Deal With Royalty Pharma | WASHINGTON (dpa-AFX) - Zymeworks Inc. (ZYME), a clinical-stage biopharmaceutical company, on Monday entered into a $250 million royalty funding agreement with Royalty Pharma plc (RPRX), a buyer... ► Artikel lesen | |
| GENUS | 33,000 | +0,61 % | Genus PLC - Director/PDMR Shareholding | ||
| ANAPTYSBIO | 47,000 | 0,00 % | AnaptysBio, Inc.: Anaptys Files Motion to Dismiss Tesaro's Claim of Anticipatory Breach of Contract in Ongoing Litigation Against Tesaro, a GSK subsidiary | Trial to resolve all claims in the Anaptys and Tesaro/GSK dispute is scheduled for July 14-17, 2026 SAN DIEGO, Jan. 08, 2026 (GLOBE NEWSWIRE) -- AnaptysBio, Inc. (Nasdaq: ANAB), a clinical-stage biotechnology... ► Artikel lesen | |
| ABIONYX PHARMA | 3,280 | -0,46 % | ABIONYX Pharma Provides an Update on Its Business and Cash Position for the 4th Quarter of 2025 | Consolidated revenue of €4.1 million at the end of December 2025 Cash position of €3.5 million as of December 31, 2025 Cash runway through year-end 2026 excluding France 2030 non-dilutive... ► Artikel lesen |
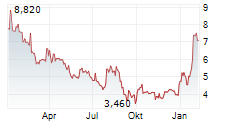
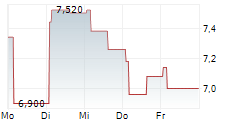
MAAT PHARMA SA gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 6,960 Euro und damit -1,69 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MAAT PHARMA SA finden Sie auf Wallstreet Online.