| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,745 | 0,790 | 17:41 | |
| 0,745 | 0,790 | 17:43 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.01. | OCUMENSION-B (01477): VOLUNTARY ANNOUNCEMENT PATIENT ENROLLMENT COMPLETED IN THE REAL-WORLD STUDY OF OT-703 IN HAINAN BOAO | 1 | HKEx | ||
| 19.12.25 | OCUMENSION-B (01477): SUPPLEMENTAL ANNOUNCEMENT | - | HKEx | ||
| 26.11.25 | OCUMENSION-B (01477): VOLUNTARY ANNOUNCEMENT APPROVAL RECEIVED FOR MARKETING OT-702 IN CHINA | 1 | HKEx | ||
| OCUMENSION THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 22.09.25 | OCUMENSION-B (01477): 2025 INTERIM REPORT | 1 | HKEx |
4 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,690 | -2,76 % | Märkte am Morgen: Bayer, SAP, Scout24, Knorr-Bremse, thyssenkrupp, Bitcoin | Der DAX hat eine turbulente Woche hinter sich. Am Freitag gab es noch einmal einen ordentlichen Schub nach oben. In den USA hatte der Supreme Court die Zölle von Donald Trump für unrechtmäßig erklärt.... ► Artikel lesen | |
| NOVO NORDISK | 31,815 | +0,44 % | Novo Nordisk enttäuscht mit Studie zu Abnehmmittel | Bagsvard - Die schlechten Nachrichten für den Pharmakonzern Novo Nordisk reissen nicht ab: Mit ihrem Abnehmmedikament Cagrisema schnitten die Dänen in einer Phase-3-Studie schlechter ab als erhofft.... ► Artikel lesen | |
| PFIZER | 23,480 | +0,38 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| MERCK KGAA | 126,30 | +0,52 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,36 | +1,81 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,170 | -3,35 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,53 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| ABBVIE | 199,60 | +1,53 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| CANOPY GROWTH | 0,929 | -1,38 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| ELI LILLY | 887,40 | -0,39 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| MERCK & CO | 104,20 | -0,57 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen | |
| ASTRAZENECA | 174,20 | -1,28 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,330 | -5,24 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? | ||
| BRISTOL-MYERS SQUIBB | 53,46 | +1,27 % | Bristol Myers Squibb: Setzt sich die relative Stärke der Pharmaaktien fort? | Nach der jüngsten Aufwärtsbewegung zeigt die Aktie von Bristol Myers Squibb eine Konsolidierung. Das Chartbild wirkt dabei weiterhin stabil Den vollständigen Artikel lesen ... ► Artikel lesen | |
| TEVA | 29,200 | +1,74 % | Teva Pharmaceutical Industries Ltd: Teva to Present at the Upcoming Investor Conferences in March | PARSIPPANY, N.J. and TEL AVIV, Israel, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) today announced that Richard Francis, Teva's President and CEO, will... ► Artikel lesen |
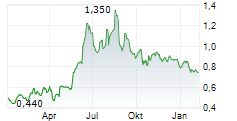
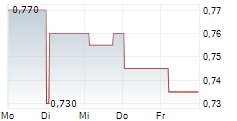
Das Unternehmen OCUMENSION THERAPEUTICS kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 0,720 Euro und damit -3,36 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu OCUMENSION THERAPEUTICS finden Sie auf Wallstreet Online.