- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Stifel initiates Palisade Bio stock with buy rating on IBD drug | 4 | Investing.com | ||
| Mo | Palisade Bio presents ulcerative colitis drug trial data at ECCO | 13 | Investing.com | ||
| PALISADE BIO Aktie jetzt für 0€ handeln | |||||
| Mo | Palisade Bio, Inc.: Palisade Bio Reports Rapid Clinical, Histologic and New Mechanistic Colon Tissue RNA Sequencing Data Supporting Targeted Activity of PALI-2108 at ECCO 2026 | 266 | GlobeNewswire (Europe) | Phase 1b translational data demonstrate localized ileocolonic target engagement, suppression of inflammatory and fibrotic gene programs, and early clinical response within seven days Encouraging safety... ► Artikel lesen | |
| 17.02. | Palisade Bio appoints IBD expert to clinical advisory board | 1 | Investing.com | ||
| 17.02. | Palisade Bio verstärkt klinischen Beirat mit CED-Spezialisten für Phase-2-Studie | 1 | Investing.com Deutsch | ||
| 17.02. | Palisade Bio, Inc.: Palisade Bio Appoints Global IBD Precision Medicine Leader Bram Verstockt, MD, PhD to Clinical Advisory Board | 1 | GlobeNewswire (USA) | ||
| 29.01. | Palisade Bio appoints two IBD experts to clinical advisory board | 3 | Investing.com | ||
| 29.01. | Palisade Bio, Inc.: Palisade Bio Appoints Leading Global IBD Experts, Laurent Peyrin-Biroulet, MD, PhD and David T. Rubin, MD, to Its Clinical Advisory Board | 267 | GlobeNewswire (Europe) | Internationally recognized leaders in inflammatory bowel disease bring deep expertise spanning ulcerative colitis, Crohn's disease, and late-stage clinical trial design Appointments strengthen Palisade... ► Artikel lesen | |
| 09.01. | B.Riley startet Coverage für Palisade Bio mit "Buy"-Rating und Kursziel von 7,00 $ | 8 | Investing.com Deutsch | ||
| 09.01. | B.Riley initiates coverage on Palisade Bio stock with Buy rating | 7 | Investing.com | ||
| 07.01. | Crohn's & Colitis Foundation investiert bis zu 500.000 US-Dollar in Palisade Bio | 4 | Investing.com Deutsch | ||
| 07.01. | Crohn's & Colitis Foundation invests up to $500,000 in Palisade Bio | 4 | Investing.com | ||
| 07.01. | Palisade Bio, Inc.: Palisade Bio Announces Strategic Equity Investment from the Crohn's & Colitis Foundation's IBD Ventures Program to Advance PALI-2108 | 136 | GlobeNewswire (Europe) | PALI-2108 is the first and only PDE4 inhibitor in development designed for targeted delivery to the terminal ileum and colon, addressing significant unmet needs in ulcerative colitis (UC) and fibrostenotic... ► Artikel lesen | |
| 30.12.25 | Palisade Bio receives patent approval in Japan for IBD drug PALI-2108 | 3 | Investing.com | ||
| 30.12.25 | Palisade Bio sichert sich Patent in Japan für IBD-Wirkstoff PALI-2108 | 2 | Investing.com Deutsch | ||
| 30.12.25 | Palisade Bio, Inc.: Palisade Bio Announces Granting of Japanese Patent Covering Composition of Matter for Lead Product Candidate, PALI-2108 | 242 | GlobeNewswire (Europe) | PALI-2108 is the first and only PDE4 inhibitor in development targetingthe terminal ileum and colon for treatment of ulcerative colitis (UC) and fibrostenotic Crohn's disease (FSCD) to address significant... ► Artikel lesen | |
| 29.12.25 | Palisade Bio: Clear Street startet Coverage mit Kaufempfehlung dank Potenzial bei Darmerkrankungen | 5 | Investing.com Deutsch | ||
| 29.12.25 | Palisade Bio stock initiated with Buy rating at Clear Street on IBD drug potential | 2 | Investing.com | ||
| 29.12.25 | Piper Sandler initiates Palisade Bio stock with Overweight rating on IBD drug | 4 | Investing.com | ||
| 29.12.25 | Palisade Bio: Piper Sandler startet Coverage mit "Overweight"-Rating dank CED-Medikament | 11 | Investing.com Deutsch |
1 2 3 Weiter >>
59 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 328,50 | -0,02 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,495 | -0,97 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,20 | -0,24 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
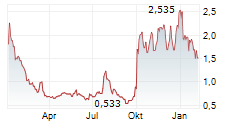
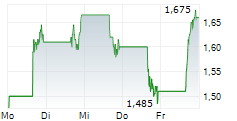
PALISADE BIO INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -4,31 % bei einem Aktienkurs von 1,775 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PALISADE BIO INC finden Sie auf Wallstreet Online.