| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
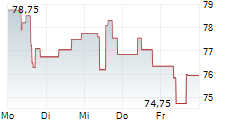
| 83,20 | 83,45 | 15:41 | |
| 83,25 | 83,50 | 15:39 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | PharmaMar 2025 presentation: revenue jumps 27%, EBITDA surges 5x | 2 | Investing.com | ||
| Fr | PHARMA MAR, S.A.: The Company sends press release regarding year 2025 results together with financial report. | 2 | CNMV | ||
| Fr | PHARMA MAR, S.A.: The Company reports draw up of the Annual Financial Statements 2025, a proposal for a dividend, and announces today's conference call with analysts and investors. | 5 | CNMV | ||
| 02.02. | PHARMA MAR, S.A.: The Company reports on treasury share acquisitions in the period from January 1 to January 31, 2026. | 5 | CNMV | ||
| 07.01. | PHARMA MAR, S.A.: The Company reports the details of the transactions executed under the Liquidity Agreement for the period from 1 October to 31 October 2025. | 3 | CNMV | ||
| 07.01. | PHARMA MAR, S.A.: The Company reports on treasury share acquisitions in the period from December 1 to December 31, 2025. | 4 | CNMV | ||
| 01.12.25 | PHARMA MAR, S.A.: The Company reports on treasury share acquisitions in the period from November 1 to November 30, 2025. | 3 | CNMV | ||
| PHARMAMAR Aktie jetzt für 0€ handeln | |||||
| 24.11.25 | PHARMA MAR, S.A.: Swiss medical authority approves PharmaMar's Zepzelca (lurbinectedin) and Atezolizumab (Tecentriq) combination as first-line maintenance therapy for extensive-stage small cell lung cancer. | 16 | CNMV | ||
| 07.11.25 | Pharmamar receives $10 million for reaching Yondelis licence agreement commercial milestone in US | 3 | thecorner.eu | ||
| 06.11.25 | PHARMA MAR, S.A.: The Company announces a US$10 million milestone payment from Janssen Products LP. | 7 | CNMV | ||
| 30.10.25 | PHARMA MAR, S.A.: The Company files third quarter 2025 financial information. | 6 | CNMV | ||
| 30.10.25 | PHARMA MAR, S.A.: The Company announces the commencement of the acquisition of treasury shares under the authorisation granted by the General Shareholders' Meeting and the temporary suspension of the existing liquidity contract. | 4 | CNMV | ||
| 16.10.25 | Pharmamar receives $50 million from Jazz Pharma for approval of Zepzelca + Tecentriq combo treatment for small cell lung cancer | 4 | thecorner.eu | ||
| 15.10.25 | PHARMA MAR, S.A.: The Company receives US$50 million milestone payment from Jazz Pharmaceuticals for the FDA's approval of Zepzelca (lurbinectedin). | 9 | CNMV | ||
| 03.10.25 | FDA approves Zepzelca (Pharma Mar) + Tecentriq (Roche) combination therapy for treating lung cancer | 17 | thecorner.eu | ||
| 03.10.25 | PHARMA MAR, S.A.: The Company reports details of the operations of the Liquidity Agreement between July 1, 2025 and September 30, 2025. | 2 | CNMV | ||
| 17.09.25 | PHARMA MAR, S.A.: Registration within the Madrid Mercantile Registry of the resolution to reduce capital by means of the redemption of own shares. | 4 | CNMV | ||
| 24.06.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 24.06.2025 | 719 | Xetra Newsboard | Das Instrument GI8 CA39032G1046 GREAT EAGLE GOLD CORP. EQUITY wird cum Kapitalmassnahme gehandelt am 24.06.2025 und ex Kapitalmassnahme am 25.06.2025 The instrument GI8 CA39032G1046 GREAT EAGLE GOLD... ► Artikel lesen | |
| 03.06.25 | PharmaMar präsentiert auf der ASCO dass Zepzelca (lurbinectedin) in Kombination mit Atezolizumab (Tecentriq) das Überleben als Erstlinien-Erhaltungstherapie bei kleinzelligem Lungenkrebs | 1.052 | news aktuell-CH | Madrid (ots/PRNewswire) - - Die Erstlinien-Erhaltungskombinationstherapie verringerte das Risiko eines Fortschreitens der Krankheit oder des Todes um 46%, mit einer medianen Gesamtüberlebenszeit von... ► Artikel lesen | |
| 03.06.25 | PharmaMar presents at ASCO that the Zepzelca(lurbinectedin) and atezolizumab (Tecentriq) combination significantly improves survival as first-line maintenance therapy for extensive-stage small cell lung cancer | 930 | PR Newswire | First-line maintenance combination therapy reduced the risk of disease progression or death by 46%, with a median overall survival of 13.2 months vs 10.6 months for atezolizumab alone from... ► Artikel lesen |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,10 | -1,29 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| EVOTEC | 5,684 | -2,97 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| BB BIOTECH | 51,60 | +0,39 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,041 | +1,50 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,625 | +0,57 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 43,340 | -4,42 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,070 | -22,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,648 | -1,73 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 330,60 | +0,62 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,300 | +23,81 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,353 | -2,62 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 328,70 | +0,21 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 163,15 | +0,49 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,690 | +0,37 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,890 | -5,25 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
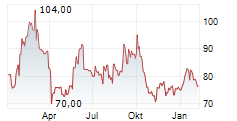
Das Unternehmen PHARMA MAR SA kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +3,56 % bei einem Aktienkurs von 82,80 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PHARMA MAR SA finden Sie auf Wallstreet Online.