| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 40,000 | 40,200 | 12:08 | |
| 40,000 | 40,200 | 11:24 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Sanofi (SNY) and Regeneron's Dupixent Recommended for EU Approval | 4 | Insider Monkey | ||
| Mo | Sanofi receives CHMP recommendation for Dupixent expansion in Europe | 7 | Pharmaceutical Technology | ||
| Mo | Sanofi receives CHMP recommendation for Dupixent approval in Europe | 7 | Pharmaceutical Technology | ||
| SANOFI SA ADR Aktie jetzt für 0€ handeln | |||||
| Mo | Sanofi: Rilzabrutinib Granted Orphan Drug Designation In Japan For IgG4-related Disease | 313 | AFX News | PARIS (dpa-AFX) - Sanofi (SNY, SAN.PA) announced the Ministry of Health, Labour and Welfare in Japan has granted orphan drug designation to rilzabrutinib, an oral, reversible covalent Bruton's... ► Artikel lesen | |
| Mo | Sanofi: Sanofi's rilzabrutinib earns orphan drug designation in Japan for IgG4-related disease | 447 | GlobeNewswire (Europe) | Sanofi's rilzabrutinib earns orphan drug designation in Japan for IgG4-related disease
Designation based on positive data from a phase 2 study of rilzabrutinib in IgG4-RDThird global orphan drug... ► Artikel lesen | |
| Fr | Sanofi, Genentech, Kedrion back star-studded bleeding disorder awareness campaign | 12 | FiercePharma | ||
| Fr | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | 444 | Dow Jones News | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| Fr | Regeneron, Sanofi Receive Positive CHMP Opinion For Dupixent In Pediatric CSU | 320 | AFX News | PARIS (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN) and Sanofi SA (SNY) Friday said the European Medicines Agencys Committee for Medicinal Products for Human Use (CHMP) has issued a positive... ► Artikel lesen | |
| Fr | Sanofi: Sanofi and Regeneron's Dupixent recommended for EU approval to treat chronic spontaneous urticaria in young children with ongoing symptoms despite treatment | 609 | GlobeNewswire (Europe) | Sanofi and Regeneron's Dupixent recommended for EU approval to treat chronic spontaneous urticaria in young children with ongoing symptoms despite treatment
If approved, Dupixent would be the first... ► Artikel lesen | |
| Fr | Sanofi's Acoziborole Receives Positive CHMP Opinion For Sleeping Sickness Treatment | 443 | AFX News | PARIS (dpa-AFX) - Sanofi SA (SNY) on Friday said the European Medicines Agency's Committee for Medicinal Products for Human Use has issued a positive opinion for Acoziborole Winthrop for the... ► Artikel lesen | |
| Fr | Sanofi's acoziborole gets EU panel backing for sleeping sickness | 4 | Investing.com | ||
| Fr | Sanofi: Acoziborole Winthrop, developed by DNDi and Sanofi, receives CHMP positive opinion as three-tablet, single-dose treatment for most common form of sleeping sickness | 434 | GlobeNewswire (Europe) | Acoziborole Winthrop, developed by DNDi and Sanofi, receives CHMP positive opinion as three-tablet, single-dose treatment for most common form of sleeping sickness
Recommendation based on phase... ► Artikel lesen | |
| Do | Sanofi Consumer Healthcare India Ltd leads gainers in 'A' group | 20 | capitalmarket.com | ||
| Do | Stock Alert: Lupin, Zydus Life, Shaily Engineering, KSB, Sanofi India, KP Energy, RVNL | 3 | capitalmarket.com | ||
| Mi | Sanofi assists Boston's World Cup plans with play for local engagement | 6 | FiercePharma | ||
| Mi | FDA Approves Sanofi And Regeneron's Dupixent As First Medicine For Allergic Fungal Rhinosinusitis | 454 | AFX News | PARIS (dpa-AFX) - Sanofi (SNY, SAN.PA) said FDA has approved Dupixent or dupilumab for the treatment of adult and pediatric patients aged 6 years and older with allergic fungal rhinosinusitis... ► Artikel lesen | |
| 24.02. | Regeneron, Sanofi Blockbuster Dupixent Scores FDA Nod For Rare Sinus Condition | 13 | Benzinga.com | ||
| 24.02. | Regeneron, Sanofi win Dupixent label expansion for allergic fungal rhinosinusitis | 5 | Seeking Alpha | ||
| 24.02. | Sanofi: Sanofi and Regeneron's Dupixent approved in the US as the first and only medicine for allergic fungal rhinosinusitis | 1.914 | GlobeNewswire (Europe) | Sanofi and Regeneron's Dupixent approved in the US as the first and only medicine for allergic fungal rhinosinusitis
Approval in adults and children aged 6 years and older supported by phase 3 study... ► Artikel lesen | |
| 23.02. | Sanofi's (SNY) R&D Track Record Remains Investor Concern, Says BofA | 30 | Insider Monkey |
1 2 3 4 5 Weiter >>
682 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,605 | -4,94 % | MustGrow Biologics und die Partnerschaft mit Bayer: Darum steht die Aktie erst am Anfang | Die globale Landwirtschaft steht vor einem Paradox. Sie muss mehr Menschen ernähren, aber mit weniger chemischen Mitteln. Weltweit sind in 162 Ländern inzwischen 460 Pestizide verboten oder eingeschränkt.... ► Artikel lesen | |
| NOVO NORDISK | 31,155 | -3,16 % | Hims & Hers nach Preisattacke auf Novo Nordisk mit Zahlen: Aktie rauscht erneut in den Keller | Die US-amerikanische Telemedizin-Plattform Hims & Hers hat am Montag nach Börsenschluss die Zahlen für das vierte Quartal respektive Gesamtjahr 2025 vorgelegt. Überschattet werden die Ergebnisse und... ► Artikel lesen | |
| PFIZER | 23,110 | -0,84 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 139,48 | -2,49 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 122,15 | -3,25 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,38 | +0,03 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,105 | -2,66 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 80,35 | -1,44 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,470 | -1,85 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 200,50 | 0,00 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 388,50 | -2,15 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,912 | -1,83 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 870,50 | 0,00 % | Eli Lilly und Novo Nordisk: Angst vor einem Preiskrieg bringt Aktien unter Druck | Novo Nordisk will ab 2027 die Preise für seine Blockbuster-Medikamente Wegovy und Ozempic in den USA drastisch senken und setzt damit Wettbewerber Eli Lilly unter Zugzwang. Die Aktien der beiden Pharmakonzerne... ► Artikel lesen | |
| MERCK & CO | 103,60 | -0,19 % | Leerink raises Merck stock price target on belzutifan growth |
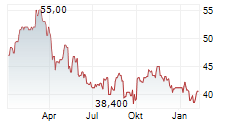
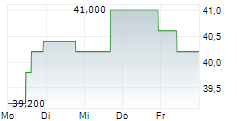
Das Unternehmen SANOFI SA ADR kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -0,98 % bei einem Aktienkurs von 40,400 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SANOFI SA ADR finden Sie auf Wallstreet Online.