| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,633 | 0,678 | 15:34 | |
| 0,633 | 0,678 | 15:34 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | SCYNEXIS Q4 Earnings Assessment | 1 | Benzinga.com | ||
| Mi | SCYNEXIS Reports Full Year 2025 Financial Results and Provides Corporate Update | 97 | GlobeNewswire (Europe) | SCYNEXIS received a one-time non-refundable payment in Q4 2025 totalling $24.8 million from GSK SCYNEXIS announced dosing of the first patient using the intravenous (IV) formulation of SCY-247 in... ► Artikel lesen | |
| Mi | SCYNEXIS INC - 10-K, Annual Report | 1 | SEC Filings | ||
| 26.02. | SCYNEXIS doses first patients in SCY-247 IV formulation trial | 3 | Investing.com | ||
| 26.02. | SCYNEXIS Announces First Participants Dosed in a Phase 1 Single Ascending Dose and Multiple Ascending Dose Trial of Intravenous SCY-247 | 99 | GlobeNewswire (Europe) | JERSEY CITY, N.J., Feb. 26, 2026 (GLOBE NEWSWIRE) -- SCYNEXIS, Inc. (NASDAQ: SCYX), a biotechnology company pioneering innovative medicines to overcome and prevent difficult-to-treat and drug-resistant... ► Artikel lesen | |
| 21.01. | JERSEY CITY - FDA grants fast track and QIDP designations to SCYNEXIS drug | 3 | Investing.com | ||
| 21.01. | SCYNEXIS Receives FDA Qualified Infectious Disease Product (QIDP) and Fast Track Designations for SCY-247 | 225 | GlobeNewswire (Europe) | The QIDP designation will ensure at least 10 years of market exclusivity for SCY-247 following approval Recent articles highlight the growing threat from a rapidly spreading, multi-drug resistant... ► Artikel lesen | |
| SCYNEXIS Aktie jetzt für 0€ handeln | |||||
| 22.12.25 | SCYNEXIS granted Nasdaq extension to regain bid price compliance | 5 | Seeking Alpha | ||
| 22.12.25 | Scynexis receives 180-day extension from Nasdaq to meet listing requirements | 4 | Investing.com | ||
| 22.12.25 | SCYNEXIS Granted 180-Day Extension by Nasdaq to Regain Compliance with Minimum Bid Price Requirement | 265 | GlobeNewswire (Europe) | JERSEY CITY, N.J., Dec. 22, 2025 (GLOBE NEWSWIRE) -- SCYNEXIS, Inc. (NASDAQ: SCYX), a biotechnology company pioneering innovative medicines to overcome and prevent difficult-to-treat and drug-resistant... ► Artikel lesen | |
| 19.11.25 | SCYNEXIS Completes Transfer of BREXAFEMME New Drug Application to GSK | 294 | GlobeNewswire (Europe) | GSK remains committed to the relaunch of BREXAFEMME, and following its relaunch, SCYNEXIS stands to receive up to $145.5 million in annual net sales milestones as well as royalties, net of payments... ► Artikel lesen | |
| 17.11.25 | Scynexis Announces Federal Funding of Collaboration Between Hackensack Meridian CDI and Johns Hopkins Researchers to Develop New Therapeutics, Including Novel Fungerps, for Resistant Fungal Infections | 2 | GlobeNewswire (USA) | ||
| 05.11.25 | SCYNEXIS Reports Third Quarter 2025 Financial Results and Provides Corporate Update | 140 | GlobeNewswire (Europe) | SCYNEXIS to receive one-time payments totalling $24.8 million from GSK as part of the resolution of the disagreement related to the restart of the Phase 3 MARIO study in invasive candidiasis. Scynexis... ► Artikel lesen | |
| 28.10.25 | Guggenheim lowers SCYNEXIS stock price target to $3 after GSK trial resolution | 25 | Investing.com | ||
| 15.10.25 | GSK makes nice with antifungal partner Scynexis with $22M payment to resolve trial dispute | 9 | FiercePharma | ||
| 15.10.25 | XFRA 135A: WIEDERAUFNAHME/RESUMPTION | 329 | Xetra Newsboard | FOLGENDE(S) INSTRUMENT(E) WIRD/ WERDEN WIEDER IN DEN HANDEL AUFGENOMMEN MIT FOLGENDEM TRADING SCHEDULE.THE FOLLOWING INSTRUMENT(S) IS/ARE RESUMED TRADING WITH FOLLOWING TRADING SCHEDULE:INSTRUMENT NAME... ► Artikel lesen | |
| 15.10.25 | SCYNEXIS erhält 22 Millionen US-Dollar von GSK nach Abbruch der MARIO-Studie | 16 | Investing.com Deutsch | ||
| 15.10.25 | XFRA 135A: AUSSETZUNG/SUSPENSION | 572 | Xetra Newsboard | DAS/ DIE FOLGENDE(N) INSTRUMENT(E) IST/ SIND AB SOFORT AUSGESETZT:THE FOLLOWING INSTRUMENT(S) IS/ ARE SUSPENDED WITH IMMEDIATE EFFECT:INSTRUMENT NAME KUERZEL/SHORTCODE ISIN BIS/UNTILSCYNEXIS INC. DL-... ► Artikel lesen | |
| 15.10.25 | SCYNEXIS and GSK Resolve Their Disagreement Related to the Restart of the Phase 3 MARIO Study | 383 | GlobeNewswire (Europe) | SCYNEXIS to receive a $22 million payment as part of the resolution related to the restart of the Phase 3 MARIO study on invasive candidiasisScynexis will promptly wind-down and terminate the MARIO... ► Artikel lesen | |
| 15.10.25 | SCYNEXIS INC - 8-K, Current Report | 1 | SEC Filings |
1 2 Weiter >>
25 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 37,795 | +1,06 % | Bayer: Kaufen, halten oder verkaufen - wie wirkt der Chart? | Bayer konsolidiert nach einer starken Aufwärtsphase derzeit im Bereich der 50-Tage-Linie. Hält diese Zone und entsteht daraus ein erneuter Impuls nach oben Den vollständigen Artikel lesen ... ► Artikel lesen | |
| NOVO NORDISK | 33,000 | +0,15 % | Novo Nordisk Aktie: Trendwende? - Auto1, Brain Biotech, Munich Re, Nordex und Rheinmetall - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| PFIZER | 22,700 | -0,68 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 138,62 | -2,30 % | HV-Termine: Hauptversammlungen bei BRAIN Biotech, LS Telcom, Metro, MVV Energie, Novartis | Einmal jährlich müssen sich Aufsichtsrat und Vorstand einer Gesellschaft den Aktionären stellen: Die Hauptversammlung ist das höchste Organ einer Aktiengesellschaft und vergleichbarer Unternehmens-Formen.... ► Artikel lesen | |
| MERCK KGAA | 119,40 | -2,97 % | Tagesvorschau 5. März: Marktausblick am Donnerstag: DHL, Merck und Aareal Bank öffnen ihre Bücher | Donnerstag: Immobilien, Industrie und WelthandelDer Donnerstag steht ganz im Zeichen großer deutscher Konzerne und wichtiger US-Konjunkturdaten. Mit Zahlen unter anderem von DHL Group, Merck KGaA, Aareal... ► Artikel lesen | |
| GILEAD SCIENCES | 125,20 | -1,68 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,115 | -1,11 % | Canadian LP Aurora Cannabis is now 'solely' a medical company | ||
| SANOFI | 77,43 | -3,42 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,330 | +0,16 % | UBS stuft GSK auf 'Neutral' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat die Einstufung für GSK mit einem Kursziel von 1940 Pence auf "Neutral" belassen. Eine Kombination der Wirkstoffe VH-184 und VH-499 gegen das... ► Artikel lesen | |
| ABBVIE | 201,00 | -0,99 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 390,00 | -2,01 % | Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament | DJ Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament
Von Adria Calatayud
DOW JONES--Der Schweizer Pharmakonzern Roche hat mit seinem Medikamentenkandidat gegen Multiple Sklerose... ► Artikel lesen | |
| CANOPY GROWTH | 0,927 | -0,96 % | Is Canopy Growth Stock Going to $0? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 852,00 | -1,17 % | Ausschreibung KONKRET-Preis 2026: Neues Setting, erhöhtes Preisgeld / Lilly Deutschland Stiftung intensiviert Innovationsförderung in der Gesundheitsversorgung | Bad Homburg (ots) - Mit dem Innovationspreis KONKRET fördert die Lilly Deutschland Stiftung Projekte, die Lücken und Barrieren in der Gesundheitsversorgung adressieren. Aufbauend auf bisherigen Erfolgen... ► Artikel lesen | |
| MERCK & CO | 101,60 | -1,74 % | Merck skizziert strategische Neuausrichtung nach KEYTRUDA-Patentablauf |
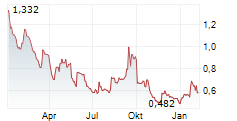
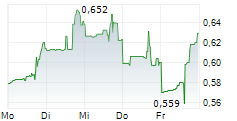
SCYNEXIS INC gehört der Branche Pharma an. Der aktuelle Kurs liegt bei 0,644 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SCYNEXIS INC finden Sie auf Wallstreet Online.