| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 2,100 | 2,190 | 13:04 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| THX PHARMA Aktie jetzt für 0€ handeln | |||||
| 20.01. | THERANEXUS: THX Pharma Announces its 2026 Financial Calendar | 307 | Actusnews Wire | Lyon, France - January 20, 2026, 18:00 p.m. CET - THX Pharma (Theranexus), an innovative biopharmaceutical company in the treatment of rare neurological diseases, today announces its financial... ► Artikel lesen | |
| 08.01. | THERANEXUS: THX Pharma announces the publication of its letter to shareholders | 384 | Actusnews Wire | Lyon, France - January 8, 2025, 6:00 PM CET - THX Pharma (Theranexus), an innovative biopharmaceutical company in the treatment of rare neurological diseases, today announces the publication of... ► Artikel lesen | |
| 07.01. | THERANEXUS: THX Pharma Announces the Appointment of Julien Veys as Deputy Chief Executive Officer | 370 | Actusnews Wire | Lyon, France - January 7, 2026, 8:00 a.m. CET - The Board of Directors of THX Pharma (Theranexus), a pharmaceutical company specializing in rare neurological diseases, today announces the appointment... ► Artikel lesen | |
| 13.11.25 | THERANEXUS: THX Pharma announces the strong success of its approximately EUR 8 million capital increase to support its continued development and strengthen its financial structure | 683 | Actusnews Wire | Capital increase of €7.8 million through the issuance of 4,356,804 new shares at a unit price of €1.80
The transaction received strong support from existing shareholders, whose demand on irreducible... ► Artikel lesen | |
| 27.10.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 27.10.2025 | 486 | Xetra Newsboard | Das Instrument XPGB NO0010736879 VEND MARKETPLAC. B NK-,50 EQUITY wird cum Kapitalmassnahme gehandelt am 27.10.2025 und ex Kapitalmassnahme am 28.10.2025 The instrument XPGB NO0010736879 VEND MARKETPLAC.... ► Artikel lesen | |
| 23.10.25 | THX Pharma announces the launch of a capital increase with preferential subscription rights of nearly EUR 8 million | 1.993 | Actusnews Wire | Subscription and guarantee commitments totaling €5.9 million, representing 75.5% of the envisaged capital increase
Subscription price: €1.80 per share, representing a 29.4% discount to the... ► Artikel lesen | |
| 14.10.25 | Thx Pharma And Exeltis Achieve Key Milestone Towards The Commercialization Of Tx01 | 603 | Actusnews Wire | THX PHARMA (THERANEXUS) AND EXELTIS ACHIEVE KEY MILESTONE TOWARDS THE COMMERCIALIZATION OF TX01
THX Pharma (Theranexus) and Exeltis have reached a pivotal milestone, setting the stage for the... ► Artikel lesen | |
| 01.10.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 01.10.2025 | 523 | Xetra Newsboard | Das Instrument RIHN CH0003671440 RIETER HLDG NA SF 5 EQUITY wird cum Kapitalmassnahme gehandelt am 01.10.2025 und ex Kapitalmassnahme am 02.10.2025 The instrument RIHN CH0003671440 RIETER HLDG NA SF... ► Artikel lesen | |
| 29.09.25 | Theranexus Becomes THX Pharma: A Strategic Evolution Toward the Commercialization of Its Medicines for Rare Diseases | 1.471 | Actusnews Wire | With TX01 and Batten-1, THX Pharma is entering a new strategic phase focused on regulatory approval, early access, and international commercialization of its medicines.
Lyon, France - September... ► Artikel lesen | |
| 25.09.25 | Theranexus Announces First Half 2025 Financial Results | 618 | Actusnews Wire | THERANEXUS ANNOUNCES FIRST HALF 2025 FINANCIAL RESULTS
Lyon, France - 25 September 2025 - 6pm CEST - Theranexus, a biopharmaceutical company innovating in the treatment of rare neurological diseases... ► Artikel lesen | |
| 10.07.25 | Theranexus Publishes Its Cash Position As Of 30 June 2025 | 612 | Actusnews Wire | Lyon, France - 10 July 2024, 6.pm CET - Theranexus, a biopharmaceutical company developing drug candidates for rare neurological diseases, today published its cash position as of 30 June 2025.
On... ► Artikel lesen | |
| 13.05.25 | Theranexus And Beyond Batten Disease Foundation Announce Strong Positive Real-world Data Supporting Batten-1 Efficacy For The Treatment Of Batten Disease | 871 | Actusnews Wire | Lyon, France - Austin, Texas, United States - Mai 13, 2025 - 6.00 pm CET - Theranexus, a biopharmaceutical company developing drug candidates for rare neurological diseases and the Beyond Batten... ► Artikel lesen | |
| 29.04.25 | Theranexus Publishes Its 2024 Full-year Results, Presents Its Progress Report And Closes Its Equity Line | 1.236 | Actusnews Wire | Lyon, France - 29 April 2025 - 7pm CET - Theranexus, a biopharmaceutical company innovating in the treatment of rare neurological diseases, today publishes its results for the year ending 31 December... ► Artikel lesen |
13 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 41,200 | -0,48 % | Morningstar, Inc.: Morningstar Completes Acquisition of CRSP and Extends Relationship with Vanguard | The CRSP integration unites two trusted sources of market insight, reinforcing a shared commitment to transparency, quality, and investor-focused solutions and solidifying Morningstar's position... ► Artikel lesen | |
| NANOREPRO | 1,535 | +2,68 % | NanoRepro ordnet Aufsichtsrat nach außerordentlicher HV neu | Die Aktionäre der NanoRepro AG haben auf der außerordentlichen Hauptversammlung vom 16. Januar 2026 den Aufsichtsrat neu zusammengesetzt. Neu gewählt wurden Gunter Heneis, Michael Herrmann und Konstantin... ► Artikel lesen |
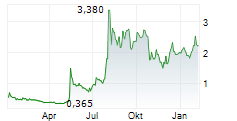
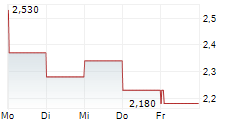
THX PHARMA SA gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,050 (-2,24 %) liegt der Kurs bei 2,180 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu THX PHARMA SA finden Sie auf Wallstreet Online.