| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 6,600 | 6,670 | 02.03. | |
| 6,620 | 6,680 | 02.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 24.02. | ADOCIA Reports Fourth Quarter 2025 Financial Results and Provides a Business Update | 309 | Business Wire | Chinese partner Tonghua Dongbao completed BioChaperone Lispro Phase 3 program with positive data on people with type 1 diabetes, completing the positive results obtained on type 2 diabetes
... ► Artikel lesen | |
| 06.02. | Number of Shares and Voting Rights of ADOCIA as of January 31st, 2026 | 281 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| ADOCIA Aktie jetzt für 0€ handeln | |||||
| 22.01. | ADOCIA Announces Half-Year Report on Adocia's Liquidity Agreement with Kepler Capital Markets | 289 | Business Wire | Regulatory News:
Under the liquidity agreement entrusted by Adocia (Euronext Paris: FR0011184241 ADOC) to Kepler Capital Markets, the following resources were listed on the liquidity account on... ► Artikel lesen | |
| 09.01. | Number of Shares and Voting Rights of ADOCIA as of December 31st, 2025 | 301 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 18.12.25 | ADOCIA Announces its Financial Calendar for 2026 | 392 | Business Wire | Regulatory News:
Adocia (Euronext Paris: FR0011184241 ADOC), a clinical-stage biopharmaceutical company focused on the research and development of innovative therapeutic solutions for the treatment... ► Artikel lesen | |
| 08.12.25 | ADOCIA SA: Number of Shares and Voting Rights of ADOCIA as of December 8th, 2025 | 344 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 04.12.25 | ADOCIA Announces the Completion of a €10 Million Fundraising | 439 | Business Wire | Fundraising of a total amount of €10 million in gross proceeds through the issuance of a total number of 1,262,626 new shares, each with one share warrant attached, subscribed by CVI Investments... ► Artikel lesen | |
| 14.11.25 | Number of Shares and Voting Rights of ADOCIA as of October 31st, 2025 | 367 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 12.11.25 | ADOCIA Announces Filing of Patent for New Long-Acting Peptides Platform in Diabetes and Obesity - AdoXLong - and Provides an Update on its BioChaperone Platform | 338 | Business Wire | New technological platform of chemical modification of acylated peptides to extend their duration of action to at least one month, AdoXLong
Patent filing with an initial application on semaglutide... ► Artikel lesen | |
| 15.10.25 | ADOCIA Reports Third Quarter 2025 Financial Results and Provides a Business Update | 509 | Business Wire | Cash position of €13.4 million as of September 30, 2025 US$10 million milestone payment from partner Tonghua Dongbao and 2024 Research Tax Credit of €2.8 million received in July 2025 ... ► Artikel lesen | |
| 15.10.25 | ADOCIA and Tonghua Dongbao Announce Positive Topline Results of Phase 3 Clinical Trial on Ultra-Rapid Insulin BioChaperone Lispro (THDB0206 injection) in people with T1D | 558 | Business Wire | This Phase 3 clinical trial on BioChaperone Lispro (THDB0206 injection) conducted in China in people with Type 1 Diabetes, successfully demonstrated, in comparison with standard of care Humalog:
... ► Artikel lesen | |
| 10.10.25 | Number of Shares and Voting Rights of ADOCIA as of September 30th, 2025 | 323 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 25.09.25 | ADOCIA Announces First Half 2025 Financial Results and Provides a Business Update | 496 | Business Wire | Cash position of €7.1m (million) as of June 30, 2025 following successful completion of €9.7m private placement in February 2025
Cash runway secured until Q2 2026 considering the US$10... ► Artikel lesen | |
| 11.09.25 | Number of Shares and Voting Rights of ADOCIA as of August 31st, 2025 | 383 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 03.09.25 | ADOCIA Announces Oral Presentations on AdoShell and BioChaperone at EASD, ESB and PODD 2025 Annual Meetings | 2.144 | Business Wire | Regulatory News:
Adocia (Euronext Paris: FR0011184241 ADOC, the "Company"), a clinical-stage biopharmaceutical company focused on the research and development of innovative therapeutic solutions... ► Artikel lesen | |
| 19.08.25 | Number of Shares and Voting Rights of ADOCIA as of July 31st, 2025 | 447 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen | |
| 25.07.25 | ADOCIA and Tonghua Dongbao Announce Positive Topline Results of Phase 3 Clinical Trial on Ultra-Rapid Insulin BioChaperone Lispro (THDB0206 injection) in People with T2D | 1.288 | Business Wire | This Phase 3 clinical trial on BioChaperone Lispro (THDB0206 injection) conducted in China in people with Type 2 diabetes, successfully demonstrated, in comparison with standard of care Humalog:
... ► Artikel lesen | |
| 23.07.25 | ADOCIA Reports Second Quarter 2025 Financial Results and Provides a Business Update | 713 | Business Wire | Cash position of €7.1 million as of June 30, 2025
US$10 million milestone payment from partner Tonghua Dongbao and 2024 Research Tax Credit of €2.8 million received in July 2025
Current... ► Artikel lesen | |
| 16.07.25 | ADOCIA Announces Half-Year Report on Adocia's Liquidity Agreement with Kepler Capital Markets | 523 | Business Wire | Regulatory News:
Under the liquidity agreement entrusted by Adocia (Euronext Paris: FR0011184241 ADOC) to Kepler Capital Markets, the following resources were listed on the liquidity account on... ► Artikel lesen | |
| 11.07.25 | Number of Shares and Voting Rights of ADOCIA as of June 30th, 2025 | 426 | Business Wire | Regulatory News:
Pursuant to the provisions of article L. 233-8 II of the French "Code de Commerce" and article 223-16 of the General Regulation of the French stock-market authorities (Autorité... ► Artikel lesen |
1 2 Weiter >>
31 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 92,30 | +0,27 % | BioNTech SE: BioNTech veröffentlicht am 10. März 2026 Ergebnisse für das vierte Quartal und das Geschäftsjahr 2025 und informiert über operativen Fortschritt | MAINZ, Deutschland, 24. Februar 2026 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, "BioNTech" oder "das Unternehmen") wird am Dienstag, den 10. März 2026, die Ergebnisse für das vierte Quartal und... ► Artikel lesen | |
| EVOTEC | 5,686 | -3,30 % | Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen | |
| BB BIOTECH | 51,60 | 0,00 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| MEDIGENE | 0,036 | -11,76 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,295 | -1,58 % | Shortseller-Positionen aktuell: freenet, GFT, HelloFresh, Hypoport, Puma, Qiagen, Redcare Pharmacy | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 45,140 | -0,15 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,070 | -17,41 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,742 | +0,08 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 329,30 | -0,18 % | AAPL, AA, BKR, and AMGN are among the top stocks for momentum, says Oppenheimer | ||
| EPIGENOMICS | 1,020 | -2,86 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,765 | +1,40 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,90 | -0,06 % | $100 Invested In Stryker 15 Years Ago Would Be Worth This Much Today | ||
| BIOGEN | 161,20 | +0,19 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,560 | -4,48 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,870 | -4,01 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
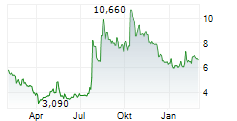
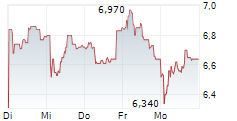
Das Unternehmen ADOCIA SAS kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 6,620 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ADOCIA SAS finden Sie auf Wallstreet Online.