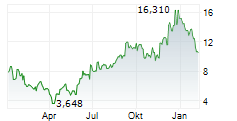
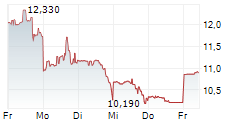
| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 11,120 | 11,400 | 07.02. | |
| 10,970 | 11,540 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | RBC Capital reiterates Outperform rating on EyePoint stock amid recent decline | 5 | Investing.com | ||
| Mo | Mizuho reiterates Outperform rating on EyePoint stock amid share weakness | 5 | Investing.com | ||
| 16.01. | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 6 | GlobeNewswire (USA) | ||
| EYEPOINT Aktie jetzt für 0€ handeln | |||||
| 07.01. | EyePoint: Mizuho bekräftigt "Outperform"-Rating und Kursziel von 33 $ | 11 | Investing.com Deutsch | ||
| 07.01. | Mizuho reiterates Outperform rating on EyePoint stock with $33 target | 1 | Investing.com | ||
| 07.01. | EyePoint's Phase 3 trials for DURAVYU in wet AMD on track for mid-2026 data | 1 | Investing.com | ||
| 07.01. | EyePoint: Phase-3-Studiendaten für DURAVYU bei feuchter AMD für Mitte 2026 erwartet | 3 | Investing.com Deutsch | ||
| 07.01. | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Corporate Update and Anticipated Pivotal Milestones for 2026 | 585 | GlobeNewswire (Europe) | - Phase 3 programs underway for DURAVYU in wet AMD and DME, the largest multi-billion-dollar retinal disease markets - - Pivotal Phase 3 trials in wet AMD on track for data readout beginning in mid-2026... ► Artikel lesen | |
| 07.01. | EyePoint, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 16.12.25 | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 8 | GlobeNewswire (USA) | ||
| 09.12.25 | EyePoint stock maintains Outperform rating at Mizuho amid competitor moves | 8 | Investing.com | ||
| 09.12.25 | Mizuho bestätigt "Outperform"-Rating für EyePoint trotz beschleunigter Konkurrenz-Pläne | 18 | Investing.com Deutsch | ||
| 08.12.25 | EyePoint Pharmaceuticals ändert Namen, Kürzel EYPT bleibt bestehen | 10 | Investing.com Deutsch | ||
| 08.12.25 | EyePoint Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 20.11.25 | EyePoint Pharmaceuticals stock maintains Buy rating at H.C. Wainwright | 6 | Investing.com | ||
| 19.11.25 | EyePoint Pharmaceuticals, Inc.: EyePoint Announces Positive Recommendation from Independent Data Safety Monitoring Committee for Pivotal Phase 3 Trials for DURAVYU in Wet Age-Related Macular Degeneration | 265 | GlobeNewswire (Europe) | - No changes in protocol recommended for LUGANO and LUCIA clinical trials - - Masked safety data continues to show no safety signals, consistent with previous clinical trials for DURAVYU - - On track... ► Artikel lesen | |
| 19.11.25 | EyePoint Pharmaceuticals, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 17.11.25 | EyePoint Pharmaceuticals, Inc.: EyePoint Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | 3 | GlobeNewswire (USA) | ||
| 12.11.25 | EyePoint Pharmaceuticals: Strategische Fortschritte in der Augenheilkunde und Finanzierung bis 2027 gesichert | 13 | Investing.com Deutsch | ||
| 10.11.25 | EyePoint stellt auf Guggenheim-Konferenz innovative Strategie zur Medikamentenfreisetzung vor | 7 | Investing.com Deutsch |
1 2 3 Weiter >>
54 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Bayer: Das wird der zukünftige Top-Seller | Der DAX-Konzern Bayer hat in den zurückliegenden Quartalen mit vielen guten Neuigkeiten aus der Pharma-Sparte bei den Anlegern punkten können. Die Leverkusener befinden sich in einer guten Position... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Hims & Hers zieht an Novo Nordisk vorbei - Der Aktienkurs legt über 10 Prozent zu! | ||
| PFIZER | 23,015 | -0,07 % | Opening Bell: Gold/Silber, PepsiCo, Merck & Co, Pfizer, PayPal, Teradyne, Uber/Snap/Alphabet, Nvidia | Die US-Börsen sind zum Wochenstart fester aus dem Handel gegangen. Der Dow Jones gewann 1,05 Prozent, der Nasdaq Composite legte 0,56 Prozent zu. Am Freitag hatte die mögliche Nominierung von Kevin... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | EQS-News: Novartis Pharma GmbH: Novartis Deutschland und das Ambulante Herzzentrum Kassel stärken ambulante Betreuung von Herzpatient*innen mit neuem Versorgungskonzept zur Überwachung von Risikofaktoren | EQS-News: Novartis Pharma GmbH
/ Schlagwort(e): Sonstiges/Sonstiges
Novartis Deutschland und das Ambulante Herzzentrum Kassel stärken ambulante Betreuung von Herzpatient*innen... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | BARCLAYS stuft MERCK KGAA auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat die Einstufung für Merck KGaA mit einem Kursziel von 130 Euro auf "Equal Weight" belassen. Der Fokus liege auf möglichen Risiken... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Abnehmpille schlägt ein: Starker Start ins neue Jahr - Novo Nordisk zeigt Eli Lilly die Rücklichter! | © Foto: Kristian Tuxen Ladegaard Berg - NurPhotoNach einem schwachen Vorjahr meldet sich Novo Nordisk 2026 eindrucksvoll zurück. Die neue Wegovy-Tablette sorgt in den USA für einen starken Marktstart.... ► Artikel lesen | |
| MERCK & CO | 103,20 | 0,00 % | Insider trades: Merck, Intel, Micron among notable names |
Das Unternehmen EYEPOINT INC kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 11,035 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EYEPOINT INC finden Sie auf Wallstreet Online.