| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 25,000 | 25,200 | 07:31 | |
| 25,000 | 25,200 | 07:31 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Transactions with shares and linked securities in Genmab A/S made by managerial employees and their closely associated persons | 2 | GlobeNewswire (USA) | ||
| Fr | Genmab A/S: Grant of Restricted Stock Units to Management and Employees and Grant of Warrants to Employees in Genmab | 2 | GlobeNewswire (USA) | ||
| Do | Genmab A/S - admittance to trading and official listing of new shares due to employee warrant exercise | 86 | GlobeNewswire | The share capital of Genmab A/S has been increased. The admittance to trading and official listing will take effect as of 27 February 2026 in the ISIN below.
ISIN:
DK0010272202
Name:
Genmab
Volume... ► Artikel lesen | |
| Di | GENMAB A/S - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| GENMAB A/S ADR Aktie jetzt für 0€ handeln | |||||
| Di | Genmab A/S: Capital Increase in Genmab as a Result of Employee Warrant Exercise | 3 | GlobeNewswire (USA) | ||
| 23.02. | Genmab A/S: Transactions in Connection with Share Buy-back Program | 5 | GlobeNewswire (USA) | ||
| 20.02. | Transactions with shares and linked securities in Genmab A/S made by managerial employees and their closely associated persons | 2 | GlobeNewswire (USA) | ||
| 18.02. | Notice to Convene the Annual General Meeting of Genmab A/S | 4 | GlobeNewswire (USA) | ||
| 18.02. | H.C. Wainwright hebt Kursziel für Genmab-Aktie wegen starker Pipeline auf 40 US-Dollar an | 14 | Investing.com Deutsch | ||
| 18.02. | H.C. Wainwright raises Genmab stock price target to $40 on pipeline strength | 6 | Investing.com | ||
| 18.02. | Genmab GAAP EPS of $15.37, revenue of $3.72B misses by $50M; initiates FY26 outlook | 26 | Seeking Alpha | ||
| 17.02. | Genmab: Q4 Earnings Insights | 4 | Benzinga.com | ||
| 17.02. | Genmab startet Aktienrückkaufprogramm im Wert von bis zu 725 Millionen DKK | 15 | Investing.com Deutsch | ||
| 17.02. | Genmab initiates share buy-back program worth up to 725 million DKK | 5 | Investing.com | ||
| 17.02. | GENMAB A/S - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 17.02. | Genmab A/S: Genmab Announces Initiation of Share Buy-Back Program | 5 | GlobeNewswire (USA) | ||
| 17.02. | Genmab FY 2025 presentation: 19% revenue growth, pipeline expansion on track | 5 | Investing.com | ||
| 17.02. | Genmab steigert Jahresumsatz 2025 um 19 % - Pipeline-Entwicklung im Plan | 8 | Investing.com Deutsch | ||
| 17.02. | Earnings Call: Genmab verfehlt Gewinnprognose für Q4 2025, Aktie legt dennoch zu | 3 | Investing.com Deutsch | ||
| 17.02. | Genmab Sees FY26 Revenue Of Up To $4.4 Bln | 406 | AFX News | WASHINGTON (dpa-AFX) - Genmab A/S (GMAB) published its 2025 annual report and projected higher revenue for 2026, supported by royalty growth and strong product sales. The biotechnology company... ► Artikel lesen |
1 2 3 4 5 Weiter >>
198 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| EVOTEC | 5,880 | 0,00 % | Evotec: Das Biotech-Drama geht in die nächste Runde | Die Aktie des Hamburger Wirkstoffforschers Evotec versucht erneut den Befreiungsschlag, doch das Misstrauen am Markt ist greifbar. Nach einer beispiellosen Serie von Rückschlägen - von Management-Skandalen... ► Artikel lesen | |
| BB BIOTECH | 50,20 | -2,33 % | BB Biotech: Gleich mehrere Zukäufe - spannende News erwartet | Im Schlussquartal 2025 hat die Schweizer Biotechgesellschaft BB Biotech einige Veränderungen am Portfolio vorgenommen. Bereits kurz nach dem Kauf wurde bei zwei Werten bereits eine Übernahme angekündigt:... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | 0,00 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| PAION | 0,090 | 0,00 % | PAION AG - Schwächesignale im Fokus | ||
| VALNEVA | 4,600 | -2,75 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 329,95 | +0,43 % | Is Amgen Stock Outperforming the Dow? | ||
| EPIGENOMICS | 1,050 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,334 | -2,84 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 327,80 | -0,06 % | Think Surgical announces first TMINI robot cases with Stryker knee implant | ||
| BIOGEN | 162,45 | +0,06 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht |
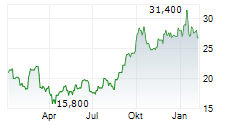
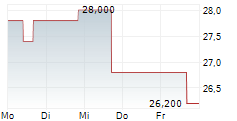
GENMAB A/S ADR gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -1,60 % bei einem Aktienkurs von 24,600 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENMAB A/S ADR finden Sie auf Wallstreet Online.