- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 27.01. | LUYE PHARMA (02186): DISCLOSEABLE AND CONNECTED TRANSACTION CAPITAL INJECTION IN NANJING LUYE | 1 | HKEx | ||
| 27.01. | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTIONS AND CONNECTED TRANSACTION FURTHER ANNOUNCEMENT ON THE TRANSFER OF 25% INTEREST IN NANJING LUYE AND RELATED ... | 1 | HKEx | ||
| 08.01. | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT NEW DRUG APPLICATION FOR CLASS 1 INNOVATIVE DRUG RUOXINLIN FOR THE NEW INDICATION OF GENERALIZED ANXIETY DISORDER ... | 2 | HKEx | ||
| 31.12.25 | LUYE PHARMA (02186): TERMS OF REFERENCE FOR NOMINATION COMMITTEE OF THE BOARD OF DIRECTORS OF THE COMPANY | 1 | HKEx | ||
| 31.12.25 | LUYE PHARMA (02186): LIST OF DIRECTORS AND THEIR ROLE AND FUNCTION | 1 | HKEx | ||
| 31.12.25 | LUYE PHARMA (02186): APPOINTMENT OF NOMINATION COMMITTEE MEMBER | 1 | HKEx | ||
| LUYE PHARMA Aktie jetzt für 0€ handeln | |||||
| 24.12.25 | Luye Pharma Grants Nhwa Rights To Commercialize Three LAI Antipsychotics In Chinese Mainland | 2 | RTTNews | ||
| 24.12.25 | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT THE GRANT OF EXCLUSIVE COMMERCIALIZATION RIGHTS TO NHWA FOR THREE LONG-ACTING INJECTABLE ANTIPSYCHOTICS IN ... | 4 | HKEx | ||
| 12.12.25 | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTION COMPLETION OF THE ISSUE OF EXCHANGEABLE PREFERENCE SHARES BY A WHOLLY-OWNED SUBSIDIARY OF THE COMPANY | 2 | HKEx | ||
| 24.11.25 | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT APPROVAL OBTAINED FOR THE GROUP'S NEW DRUG LY03017 FOR CLINICAL TRIALS IN THE U.S. | 2 | HKEx | ||
| 21.11.25 | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTION ISSUE OF EXCHANGEABLE PREFERENCE SHARES BY A WHOLLY-OWNED SUBSIDIARY OF THE COMPANY | 1 | HKEx | ||
| 29.09.25 | LUYE PHARMA (02186): INTERIM REPORT 2025 | 6 | HKEx | ||
| 09.09.25 | LUYE PHARMA (02186): NEXT DAY DISCLOSURE RETURN | 2 | HKEx | ||
| 09.09.25 | LUYE PHARMA (02186): OVERSEAS REGULATORY ANNOUNCEMENT | 2 | HKEx |
14 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,685 | -2,77 % | DAX schwächer, Gold, Silber stark: Zollsorgen, Hella, Siemens, Bayer, Rheinmetall, Hochtief ... | Der DAX hat sich am Freitag freundlich ins Wochenende verabschiedet. Er stieg am Ende 0,9 Prozent auf 25.260,69 Zähler. Der Start in die neue Woche sieht allerdings deutlich schwächer aus. Zollunsicherheit... ► Artikel lesen | |
| NOVO NORDISK | 31,735 | +0,19 % | Novo Nordisk: Nächster Nackenschlag | Im aufstrebenden Adipositas-Markt droht der dänische Biopharma-Riese Novo Nordisk weiter hinter den US-Wettbewerber Eli Lilly zurückzufallen. Neue Daten zum Hoffnungsträger CagriSema sorgen erneut für... ► Artikel lesen | |
| PFIZER | 23,460 | +0,30 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,72 | +0,41 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 126,35 | +0,56 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,36 | +1,81 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,170 | -3,35 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,53 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,870 | +0,24 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 199,60 | +1,53 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 395,85 | -1,92 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,929 | -1,38 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 887,40 | -0,39 % | Rückschlag im Abnehm-Boom: Lilly schlägt Novo deutlich | Der globale Markt für Abnehmmedikamente wächst rasant - doch der Wettbewerb verschärft sich. Neue Studiendaten setzen einen der führenden Anbieter spürbar unter Druck. Für Anleger rückt damit die Frage... ► Artikel lesen | |
| MERCK & CO | 104,00 | -0,76 % | US-Konzern Merck & Co will Pharmasparte aufspalten | RAHWAY (dpa-AFX) - Der US-Konzern Merck & Co spaltet seine Pharmasparte auf. Künftig soll das Geschäft mit Krebsmedikamenten als eigener Bereich geführt werden, teilte das Unternehmen am Montag mit.... ► Artikel lesen |
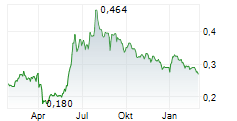
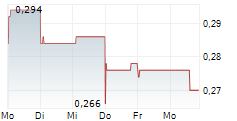
LUYE PHARMA GROUP LTD gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von -0,006 (-2,17 %) liegt der Kurs bei 0,270 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LUYE PHARMA GROUP LTD finden Sie auf Wallstreet Online.