| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 13,100 | 14,100 | 03.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Pharming Group Gears Up to Report Q4 Earnings: What's in the Cards? | 2 | Zacks | ||
| PHARMING GROUP NV ADR Aktie jetzt für 0€ handeln | |||||
| Do | Pharming Group N.V.: Pharming Group to report fourth quarter and full year 2025 financial results and provide business update on March 12 | 687 | GlobeNewswire (Europe) | Leiden, the Netherlands, February 26, 2026: Pharming Group N.V. ("Pharming" or "the Company") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) confirms that it will report its preliminary (unaudited) financial... ► Artikel lesen | |
| 17.02. | Pharming Group N.V.: Pharming Group to present at the Oppenheimer 36th Annual Healthcare Life Sciences Conference | 374 | GlobeNewswire (Europe) | Leiden, the Netherlands, February 17, 2026: Pharming Group N.V. ("Pharming" or "the Company") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) today announced its participation in the Oppenheimer 36th Annual... ► Artikel lesen | |
| 03.02. | Pharming Group N.V.: Pharming Group announces 2026 financial guidance and highlights rare disease pipeline at Investor Day | 760 | GlobeNewswire (Europe) | Highlights advancing clinical-stage pipeline, including two major value-creating programs for primary immunodeficiencies (PIDs) with immune dysregulation and mtDNA-driven mitochondrial diseaseIntroduces... ► Artikel lesen | |
| 02.02. | With FDA rejections, Aquestive's shares go up, while Pharming's go down | 16 | FiercePharma | ||
| 02.02. | FDA Strikes Off European Biotech Pharming's Immune Deficiency Drug For Expanded Use In Pediatric Patients | 13 | Benzinga.com | ||
| 02.02. | FDA knocks back Pharming's bid for wider Joenja use | 6 | pharmaphorum | ||
| 02.02. | Pharming Group N.V. - 6-K, Report of foreign issuer | 7 | SEC Filings | ||
| 02.02. | Pharming Group Receives FDA Complete Response Letter For Joenja Pediatric SNDA | 12 | RTTNews | ||
| 01.02. | Pharming Group N.V.: Pharming Group receives Complete Response Letter from U.S. FDA for sNDA for Joenja (leniolisib) in children aged 4 to 11 years with APDS | 627 | GlobeNewswire (Europe) | Leiden, the Netherlands, February 1, 2026: Pharming Group (Euronext: PHARM; Nasdaq: PHAR) today announced that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL)... ► Artikel lesen | |
| 21.01. | Original-Research: Pharming Group NV (von First Berlin Equity Research GmbH): Buy | 388 | EQS Group (EN) | Original-Research: Pharming Group NV - from First Berlin Equity Research GmbH
21.01.2026 / 16:13 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer... ► Artikel lesen | |
| 08.01. | Pharming Group N.V. - 6-K, Report of foreign issuer | 11 | SEC Filings | ||
| 08.01. | Pharming Group Sees Higher-than-Expected Revenues In Fiscal 2025 | 6 | RTTNews | ||
| 08.01. | Pharming Group N.V.: Pharming Group reports preliminary 2025 revenues and announces Investor Day | 581 | GlobeNewswire (Europe) | Leiden, The Netherlands, January 8, 2026: Pharming Group N.V. ("Pharming" or "the Company") (EURONEXT Amsterdam: PHARM/Nasdaq: PHAR) today announced its preliminary, unaudited revenues for the full... ► Artikel lesen | |
| 08.12.25 | Pharming Group N.V.: Pharming Group to participate in Oppenheimer Movers in Rare Disease Summit | 396 | GlobeNewswire (Europe) | Leiden, the Netherlands, December 8, 2025: Pharming Group N.V. ("Pharming") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) today announced its participation in the Oppenheimer Movers in Rare Disease Summit... ► Artikel lesen | |
| 06.11.25 | Pharming Group N.V. - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 06.11.25 | Pharming GAAP EPS of $0.01 beats by $0.01, revenue of $97.3M beats by $4.46M | 6 | Seeking Alpha | ||
| 06.11.25 | Pharming Group N.V.: Pharming Group reports third quarter 2025 financial results with significant growth in revenue, profitability and cash flow | 596 | GlobeNewswire (Europe) | Total third quarter 2025 revenues increased by 30% to US$97.3 million, compared to third quarter 2024RUCONEST® third quarter revenue increased by 29% to US$82.2 million, compared to third quarter 2024... ► Artikel lesen | |
| 30.10.25 | Pharming Group N.V.: Pharming Group to participate in Fireside Chat at Jefferies Global Healthcare Conference in London | 508 | GlobeNewswire (Europe) | Leiden, the Netherlands, October 30, 2025: Pharming Group N.V. ("Pharming") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) announces that Pharming management will participate in the Jefferies Global Healthcare... ► Artikel lesen | |
| 23.10.25 | Pharming Group N.V.: Pharming Group to report third quarter 2025 financial results and provide business update on November 6 | 460 | GlobeNewswire (Europe) | Leiden, the Netherlands, October 23, 2025: Pharming Group N.V. ("Pharming") (Euronext Amsterdam: PHARM/Nasdaq: PHAR) confirms that it will report its preliminary (unaudited) financial results for the... ► Artikel lesen |
1 2 3 Weiter >>
48 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| INTELLIA THERAPEUTICS | 11,850 | +1,20 % | Intellia Therapeutics, Inc.: Intellia Therapeutics Announces FDA Lift of Clinical Hold on MAGNITUDE Phase 3 Clinical Trial in ATTR-CM | CAMBRIDGE, Mass., March 02, 2026 (GLOBE NEWSWIRE) -- Intellia Therapeutics, Inc. (Nasdaq: NTLA), a leading biopharmaceutical company focused on revolutionizing medicine leveraging CRISPR gene editing... ► Artikel lesen | |
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,326 | +1,01 % | PacBio and DNAstack Collaborate on Global HiFi Genome Network Hub | ||
| ATAIBECKLEY | 3,200 | -1,23 % | XETR NEW INSTRUMENTS AVAILABLE ON XETRA - 25.02.2026 | Das Instrument B72 US04650F1012 ATAIBECKLEY INC. O.N. EQUITY hat seinen ersten Handelstag am 25.02.2026: CONTINUOUS TRADING WITH AUCTIONS, PAG NAM0, SettlCurr EUR, CCP Y
The instrument B72 US04650F1012... ► Artikel lesen | |
| BIOMERIEUX | 97,90 | -1,76 % | BioMerieux SA Profit Declines In Full Year | PARIS (dpa-AFX) - BioMerieux SA (EYWN.MU) reported earnings for full year that Drops, from the same period last yearThe company's bottom line totaled EUR397.5 million, or EUR3.34 per share.... ► Artikel lesen | |
| LIR LIFE SCIENCES | 0,596 | +1,71 % | EQS-Media: First-Mover mit erheblichem Skalierungspotenzial: LIR Life Sciences setzt Innovations-Roadmap fort | EQS-Media / 06.02.2026 / 16:20 CET/CEST
LIR Life Sciences Corp. (ISIN: CA50206C1005 | WKN: A41QA9), LIR oder das Unternehmen, freut sich bekannt zu geben, dass es mit "Neuland Laboratories Limited"... ► Artikel lesen | |
| BIO GREEN MED SOLUTION | 1,020 | 0,00 % | Bio Green Med Solution, Inc.: Bio Green Med Solution Reports Third Quarter Financial Results and Provides Business Update | KUALA LUMPUR, Malaysia, Nov. 13, 2025 (GLOBE NEWSWIRE) -- Bio Green Med Solution, Inc. (NASDAQ: BGMS, NASDAQ: BGMSP; "BGMS" or the "Company" (formerly Cyclacel Pharmaceuticals, Inc.)), a diversified... ► Artikel lesen | |
| GENUS | 33,000 | +0,61 % | Genus PLC - Director/PDMR Shareholding | ||
| ANAPTYSBIO | 47,000 | +3,07 % | AnaptysBio GAAP EPS of $1.58 beats by $0.71, revenue of $108.25M beats by $21.16M | ||
| ABIONYX PHARMA | 3,280 | -0,46 % | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | Article L233-8-II of the French Commercial Code Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| XTL BIOPHARMACEUTICALS | 0,630 | 0,00 % | XTL Biopharmaceuticals Ltd.: XTL Announces Receipt of Staff Delist Determination from Nasdaq and Plans to Request Hearing | RAMAT GAN, ISRAEL, Feb. 27, 2026 (GLOBE NEWSWIRE) -- XTL Biopharmaceuticals Ltd. (Nasdaq:XTLB) (TASE:XTLB.TA) (the "Company" or "XTL"), announced today that it has received a letter (the "Letter")... ► Artikel lesen | |
| AKESO | 10,600 | -1,85 % | Akeso, Inc.: Cadonilimab (PD-1/CTLA-4) Receives FDA Clearance for Global Phase III First-Line Gastric Cancer Trial Versus Nivolumab | HONG KONG, Dec. 11, 2025 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso" or the "Company") announced FDA approval to initiate COMPASSION-37/AK104-311 trial, a global... ► Artikel lesen | |
| MEIRAGTX | 6,500 | +1,56 % | MeiraGTx Reports Third Quarter 2025 Financial and Operational Results | Entered into broad strategic collaboration with Eli Lilly and Company ("Lilly") in the area of ophthalmology, granting Lilly worldwide exclusive rights to the Company's AAV-AIPL1 program for treatment... ► Artikel lesen | |
| MAAT PHARMA | 6,840 | -3,39 % | MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | Article 223-16 of the General Regulations of the Financial Markets Authority
(AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| NEUMORA THERAPEUTICS | 3,210 | 0,00 % | Neumora Therapeutics, Inc.: Neumora Therapeutics Highlights 2026 Pipeline Strategy and Anticipated Upcoming Milestones | Multiple clinical data readouts expected in 2026 present opportunity for substantial value creation KOASTAL-2 and -3 on track for consolidated topline readout in the second quarter of 2026 Plans to... ► Artikel lesen | |
| ISOFOL MEDICAL | 0,052 | +1,76 % | Isofol Medical AB: Isofol provides update on the ongoing phase Ib/II clinical study of arfolitixorin | GOTHENBURG, Sweden, February 24, 2026 - Isofol Medical AB (publ) (Nasdaq Stockholm: ISOFOL) today provides an update on the company's ongoing phase Ib/II clinical study of arfolitixorin, which is initially... ► Artikel lesen |
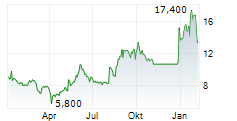
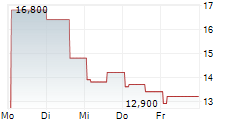
Das Unternehmen PHARMING GROUP NV ADR kann der Branche Biotechnologie zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,200 (-1,50 %) liegt der Kurs bei 13,100 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PHARMING GROUP NV ADR finden Sie auf Wallstreet Online.