| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
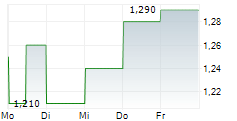
| 1,320 | 1,360 | 28.02. | |
| 1,290 | 1,400 | 27.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | SIMCERE PHARMA (02096): PROFIT ALERT | 1 | HKEx | ||
| SIMCERE PHARMACEUTICAL Aktie jetzt für 0€ handeln | |||||
| 03.02. | SIMCERE PHARMA (02096): VOLUNTARY ANNOUNCEMENT - RECEIPT OF RECENT MILESTONE PAYMENT UNDER SIM0500 OPTION TO LICENSE AGREEMENT WITH ABBVIE | - | HKEx | ||
| 02.02. | Boehringer Ingelheim and Simcere announce inflammatory bowel disease collaboration | 8 | PMLiVE | ||
| 28.01. | EQS-News: Simcere Pharmaceutical Group Limited: Boehringer Ingelheim und Simcere gehen Partnerschaft ein, um eine dual-zielgerichtete Antikörperbehandlung zur Behandlung von entzündlichen Darmerkrankungen voranzutreiben | 350 | EQS Group (DE) | EQS-News: Simcere Pharmaceutical Group Limited
/ Schlagwort(e): Vereinbarung/Vertrag
Boehringer Ingelheim und Simcere gehen Partnerschaft ein, um eine dual-zielgerichtete Antikörperbehandlung... ► Artikel lesen | |
| 27.01. | Boehringer Ingelheim, Simcere Partner on Antibody Treatment for IBD | 2 | Contract Pharma | ||
| 27.01. | Boehringer pens €1.05B deal for Simcere's preclinical IBD bispecific | 5 | FierceBiotech | ||
| 27.01. | Boehringer signs €1bn+ deal for Simcere IBD candidate | 3 | pharmaphorum | ||
| 27.01. | Simcere Pharmaceutical Group Limited: Boehringer Ingelheim and Simcere partner to advance a dual-target antibody treatment to address unmet needs in inflammatory bowel disease | 1.144 | PR Newswire | Novel TL1A/IL23p19 bispecific antibody targets drivers of disease pathogenesis to overcome the efficacy ceiling in inflammatory bowel disease. License and collaboration agreement strengthen... ► Artikel lesen | |
| 27.01. | Boehringer Ingelheim Limited: Boehringer Ingelheim and Simcere partner to advance a dual-target antibody treatment to address unmet needs in inflammatory bowel disease | 531 | GlobeNewswire (Europe) | Novel TL1A/IL23p19 bispecific antibody targets drivers of disease pathogenesis to overcome the efficacy ceiling in inflammatory bowel disease. License and collaboration agreement strengthen Boehringer's... ► Artikel lesen | |
| 27.01. | SIMCERE PHARMA (02096): VOLUNTARY ANNOUNCEMENT - ENTERING INTO EXCLUSIVE LICENSING AGREEMENT WITH BOEHRINGER INGELHEIM IN RELATION TO SIM0709 (TL1A/IL23P19 ... | 3 | HKEx | ||
| 20.01. | SIMCERE PHARMA (02096): NEXT DAY DISCLOSURE RETURN | 1 | HKEx | ||
| 12.01. | SIMCERE PHARMA (02096): NEXT DAY DISCLOSURE RETURN | 2 | HKEx | ||
| 09.01. | SIMCERE PHARMA (02096): PROPOSED SPIN-OFF AND SEPARATE LISTING OF SIMCERE ZAIMING PHARMACEUTICAL CO., LTD. ON THE MAIN BOARD OF THE STOCK EXCHANGE OF ... | - | HKEx | ||
| 06.01. | Simcere eyes growing pharmaceutical niche | 1 | China Daily | ||
| 23.12.25 | Ipsen outlays $1bn for China-based Simcere's preclinical ADC | 1 | Pharmaceutical Technology | ||
| 23.12.25 | SIMCERE PHARMA (02096): VOLUNTARY ANNOUNCEMENT - THE CLINICAL TRIAL APPROVAL FOR SIM0610 (EGFR/CMET BISPECIFIC ANTIBODY-DRUG CONJUGATE) ISSUED BY THE ... | 2 | HKEx | ||
| 22.12.25 | Ipsen licenses Simcere ADC in $1bn-plus deal | 3 | pharmaphorum | ||
| 22.12.25 | Ipsen continues ADC push with $1B deal for Simcere's preclinical LRRC15-targeting asset | 1 | FierceBiotech | ||
| 22.12.25 | Ipsen Pharma: Ipsen expands early development pipeline with Simcere Zaiming's innovative antibody drug conjugate | 663 | GlobeNewswire (Europe) | Ipsen gains exclusive global rights, outside of Greater China, for development, manufacturing and commercialization of SIM0613, a LRRC15-targeting antibody-drug conjugateSIM0613 is optimally designed... ► Artikel lesen | |
| 22.12.25 | Simcere Pharma Licenses SIM0613 To Ipsen | 604 | AFX News | BEIJING (dpa-AFX) - Simcere Pharmaceutical Group Ltd. (2096.HK, SMHGF), on Monday, announced that a subsidiary, Jiangsu Simcere Zaiming Pharmaceutical Co., Ltd., has entered into an exclusive... ► Artikel lesen |
1 2 3 Weiter >>
45 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Bayer Aktie: Kursrally am absoluten Limit - droht jetzt der große Rücksetzer? | © Foto: sbDie Bayer-Aktie hat in kurzer Zeit eine beeindruckende Aufholjagd hingelegt. Vom Tief bei unter 20 Euro schoss der Kurs auf knapp 50 Euro - mehr als eine Verdopplung. Klingt gut, aber genau... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Novo Nordisk enttäuscht mit Abnehmspritze CagriSema - Eli Lillys Präparat überlegen | Der dänische Pharmakonzern Novo Nordisk hat mit seinem Kombipräparat CagriSema das primäre Ziel der Phase-3-Studie REDEFINE 4 verfehlt. Das Medikament erreichte nach 84 Wochen zwar einen Gewichtsverlust... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| MERCK KGAA | 128,60 | +2,35 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,41 | -0,40 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 25,040 | +0,93 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| CANOPY GROWTH | 0,950 | +0,85 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| ELI LILLY | 889,90 | -0,11 % | Novo posts worst monthly decline as Lilly extends lead | ||
| MERCK & CO | 104,60 | -0,19 % | US-Konzern Merck & Co will Pharmasparte aufspalten | RAHWAY (dpa-AFX) - Der US-Konzern Merck & Co spaltet seine Pharmasparte auf. Künftig soll das Geschäft mit Krebsmedikamenten als eigener Bereich geführt werden, teilte das Unternehmen am Montag mit.... ► Artikel lesen | |
| ASTRAZENECA | 177,25 | +0,45 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,670 | -0,15 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? | ||
| BRISTOL-MYERS SQUIBB | 52,90 | +0,21 % | Bristol Myers Squibb: Setzt sich die relative Stärke der Pharmaaktien fort? | Nach der jüngsten Aufwärtsbewegung zeigt die Aktie von Bristol Myers Squibb eine Konsolidierung. Das Chartbild wirkt dabei weiterhin stabil Den vollständigen Artikel lesen ... ► Artikel lesen |
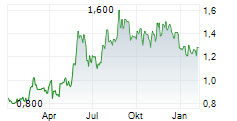
SIMCERE PHARMACEUTICAL GROUP LTD gehört der Branche Pharma an. Der aktuelle Kurs liegt bei 1,300 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SIMCERE PHARMACEUTICAL GROUP LTD finden Sie auf Wallstreet Online.