| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 30,420 | 30,560 | 17:07 | |
| 30,420 | 30,500 | 17:07 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 07:12 | BioArctic: BLA for subcutaneous formulation of Leqembi designated for Priority Review in China | 440 | PR Newswire | STOCKHOLM, Feb. 9, 2026 /PRNewswire/ -- BioArctic AB's (publ) (NASDAQ: BIOA B (Stockholm: BIOA B) partner Eisai announced today that the Biologics License Application (BLA) for the treatment... ► Artikel lesen | |
| Fr | BioArctic AB: Sales of Leqembi totaled 20.7 billion yen in the fourth quarter 2025 | 272 | GlobeNewswire (Europe) | Stockholm, Sweden, February 6, 2026 - BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai today published the preliminary global revenue for Leqembi for the fourth quarter 2025, in conjunction... ► Artikel lesen | |
| 26.01. | BioArctic: Eisai submits Marketing Authorisation Variation to EMA for intravenous maintenance dosing every four weeks with Leqembi (lecanemab) | 414 | PR Newswire | STOCKHOLM, Sweden, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that they have submitted a proposed Marketing Authorisation... ► Artikel lesen | |
| 26.01. | BioArctic's Partner Eisai Receives Priority Review For Leqembi Iqlik Subcutaneous Autoinjector | 300 | AFX News | TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics... ► Artikel lesen | |
| 26.01. | BioArctic: Leqembi Iqlik (lecanemab-irmb) supplemental Biologics License Application regarding subcutaneous starting dose granted Priority Review by the US FDA | 642 | PR Newswire | STOCKHOLM, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (STO: BIOA B) partner Eisai announced today that the supplemental Biologics License Application (sBLA) for Leqembi Iqlik subcutaneous... ► Artikel lesen | |
| 06.01. | BioArctic: BLA for subcutaneous formulation of Leqembi accepted in China | 394 | PR Newswire | STOCKHOLM, Jan. 6, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that the Biologics License Application (BLA) for the subcutaneous formulation... ► Artikel lesen | |
| 09.12.25 | BioArctic: Leqembi included in China's commercial insurance innovative drug list | 443 | PR Newswire | STOCKHOLM, Dec. 8, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Leqembi (lecanemab), has been included in the "Commercial Insurance... ► Artikel lesen | |
| 04.12.25 | BioArctic: New Leqembi-data presented at CTAD 2025 suggests potential to delay disease progression by up to 8.3 years with continued treatment | 453 | PR Newswire | STOCKHOLM, Dec. 4, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai presented the latest findings on time saved with continued treatment with lecanemab... ► Artikel lesen | |
| BIOARCTIC Aktie jetzt für 0€ handeln | |||||
| 02.12.25 | AKTIONÄR-Hot-Stock BioArctic: Debatte um Alzheimer-Medikament - so reagiert die Aktie | 489 | Der Aktionär | Seit dem September ist das Alzheimer-Medikament Leqembi, welches seinen Ursprung in Schweden bei BioArctic hat, auch in Deutschland zugelassen. Ein nun veröffentlichter Report sieht keinen Beweis für... ► Artikel lesen | |
| 19.11.25 | BioArctic: New data on lecanemab to be presented at CTAD conference | 697 | PR Newswire | STOCKHOLM, Nov. 18, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai will present the latest findings on lecanemab (Leqembi®) at the Clinical Trials... ► Artikel lesen | |
| 14.11.25 | BioArctic: Leqembi approved for IV maintenance treatment in the United Kingdom | 494 | PR Newswire | STOCKHOLM, Nov. 13, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Leqembi (lecanemab) has been approved for once every four... ► Artikel lesen | |
| 13.11.25 | Interim Report for the period July - September 2025: BioArctic | 359 | PR Newswire | STOCKHOLM, Nov. 13, 2025 /PRNewswire/ --
Broadening our portfolio with new projects and partnerships
Events during the third quarter 2025
New... ► Artikel lesen | |
| 04.11.25 | Invitation to presentation of BioArctic's third quarter report for July - September 2025 on November 13 at 9.30 a.m. CET | 346 | PR Newswire | STOCKHOLM, Nov. 4, 2025 /PRNewswire/ -- BioArctic AB (publ) (Nasdaq Stockholm: BIOA B) will publish the company's third quarter report for July - September 2025 on Thursday, November... ► Artikel lesen | |
| 30.10.25 | BioArctic: Sales of Leqembi totaled 18 billion yen in the third quarter 2025 | 336 | PR Newswire | STOCKHOLM, Oct. 30, 2025 /PRNewswire/ -- BioArctic AB's (publ) (STO: BIOA B) partner Eisai today published the preliminary global revenue for Leqembi during the third quarter 2025... ► Artikel lesen | |
| 27.10.25 | BioArctic: Health Canada Grants Authorization for Leqembi (lecanemab) | 358 | PR Newswire | STOCKHOLM, Oct. 27, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Health Canada has issued a Notice of Compliance with Conditions... ► Artikel lesen | |
| 18.10.25 | BioArctic stellt Nominierungsausschuss vor | 3 | Windkraft-Journal | ||
| 14.10.25 | BioArctic: First patient treated with Leqembi (lecanemab) in the Nordics | 348 | PR Newswire | STOCKHOLM, Oct. 14, 2025 /PRNewswire/ -- BioArctic AB (publ) (NASDAQ Stockholm: BIOA B) announced today that Leqembi has now been made available at a private clinic in Finland and... ► Artikel lesen | |
| 14.10.25 | BioArctic: Leqembi Iqlik (lecanemab-irmb) selected by TIME as one of the best innovations of 2025 | 413 | PR Newswire | STOCKHOLM, Oct. 14, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that Leqembi Iqlik, a subcutaneous autoinjector formulation... ► Artikel lesen | |
| 07.10.25 | BioArctic: Leqembi Iqlik (lecanemab-irmb) maintenance treatment launched in the U.S. | 377 | PR Newswire | STOCKHOLM, Oct. 6, 2025 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that lecanemab-irmb subcutaneous injection (U.S. brand name:... ► Artikel lesen | |
| 01.10.25 | AKTIONÄR-Hot-Stock BioArctic: Nächste Zulassungen | 477 | Der Aktionär | Das neuartige Alzheimer-Medikament Leqembi (Lecanemab) steht immer mehr Menschen rund um den Globus zur Verfügung. Fortan darf das Mittel auch in Australien und in einer weiteren Darreichungsform in... ► Artikel lesen |
1 2 3 4 Weiter >>
67 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 91,60 | +1,50 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,170 | +1,15 % | Zink-Boom, Turnaround, Biotech-Wachstum: So profitieren Sie mit Pasinex Resources, Puma und Evotec | In volatilen Märkten suchen Anleger nach außergewöhnlichen Chancen. Drei Unternehmen stechen dabei heraus. Ein Rohstoffunternehmen mit außergewöhnlichen Zink-Projekten, ein Sportartikelhersteller im... ► Artikel lesen | |
| BB BIOTECH | 50,10 | -1,76 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 42,730 | -1,71 % | Diagnostikkonzern Qiagen überrascht im Schlussquartal - Weiteres Wachstum 2026 | VENLO/HILDEN (dpa-AFX) - Qiagen hat 2025 von einer anziehenden Nachfrage nach seinen Kernprodukten profitiert. Dabei lief das Schlussquartal besser als vom Labordienstleister und Diagnostikkonzern... ► Artikel lesen | |
| MODERNA | 34,900 | +0,65 % | Moderna to Report Q4 Earnings: Is a Beat in Store for the Stock? | ||
| PAION | 0,190 | +2,70 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,076 | +2,88 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 315,40 | -2,94 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,840 | -3,45 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 7,009 | +0,62 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 296,90 | -2,05 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 165,40 | +4,52 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,370 | -3,27 % | Biofrontera's Ameluz shows strong results in actinic keratosis trial | ||
| HEIDELBERG PHARMA | 2,810 | -3,44 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
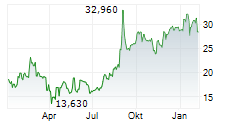
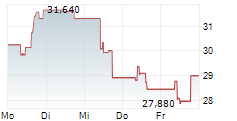
Das Unternehmen BIOARCTIC AB kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +4,41 % bei einem Aktienkurs von 30,280 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIOARCTIC AB finden Sie auf Wallstreet Online.