- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 16.12.25 | SenzaGen AB: SenzaGen further strengthens US market position with 1.5 MSEK order from global chemical industry leader | 103 | GlobeNewswire (Europe) | SenzaGen continues to solidify its role as an important partner for customers with high safety testing requirements, securing an assignment worth approximately 1.5 MSEK from a leading American chemical... ► Artikel lesen | |
| 20.11.25 | SenzaGen AB: SenzaGen opens expanded laboratory facilities to strengthen capacity in line with 2030 growth plan | 107 | GlobeNewswire (Europe) | To support its recently announced growth strategy through 2030, SenzaGen has opened expanded office and laboratory facilities in Lund. This investment strengthens the company's ability to meet the growing... ► Artikel lesen | |
| 18.11.25 | SenzaGen AB: UK signals shift to non-animal testing - new opportunities for SenzaGen | 124 | GlobeNewswire (Europe) | The UK government has recently unveiled a national strategy to accelerate the phase-out of animal testing in research and product development. The plan includes clear timelines and governance to ensure... ► Artikel lesen | |
| SENZAGEN Aktie jetzt für 0€ handeln | |||||
| 05.11.25 | SenzaGen AB: SenzaGen's interim report January-September 2025 | Growth and break-even in Q3 | 110 | GlobeNewswire (Europe) | Message from the CEO"We delivered a strong third quarter in line with our expectations, with higher sales and significantly improved EBITDA, both sequentially and year on year, which I am pleased to... ► Artikel lesen | |
| 22.08.25 | SenzaGen AB: SenzaGen secures new follow-up order worth approx. 1.0 MSEK for GARD from a global leading U.S. pharmaceutical company | 179 | GlobeNewswire (Europe) | SenzaGen has received a new follow-up order from a global leader in the pharmaceutical and life science industry. The order, valued at approximately 1.0 million SEK, covers testing with GARD®air, SenzaGen's... ► Artikel lesen | |
| 20.08.25 | SenzaGen AB: SenzaGen's interim report January-June 2025 | Strong order intake and expanded OECD approval for GARDskin | 281 | GlobeNewswire (Europe) | Message from the CEO
"We continue to win new customers, and our order intake is significantly higher than our sales for the period, giving us a strong starting position for the coming quarters. Given... ► Artikel lesen | |
| 30.06.25 | SenzaGen AB: SenzaGen obtains expanded OECD approval for GARDskin - further strengthens position in non-animal regulatory testing | 284 | GlobeNewswire (Europe) | SenzaGen's non-animal test method GARD®skin, which has been approved since 2022 under OECD Test Guideline TG 442E, has now also been included in the newly introduced OECD TG 497. This international... ► Artikel lesen | |
| 24.06.25 | SenzaGen AB: SenzaGen receives new assignment worth 1.5 MSEK from RIFM for continued collaboration in photosensitization testing | 186 | GlobeNewswire (Europe) | SenzaGen has been awarded a new grant worth 1.5 MSEK from the Research Institute for Fragrance Materials (RIFM), a leading international research institute in fragrance safety. The assignment involves... ► Artikel lesen | |
| 14.05.25 | SenzaGen AB: Report from AGM of SenzaGen AB on 14 May 2025 | 260 | GlobeNewswire (Europe) | The Annual General Meeting (AGM) of SenzaGen held on today's date, 14 May 2025 in Lund, resolved to pass all proposals presented by the board and shareholders.
Adoption of income statement and balance... ► Artikel lesen | |
| 14.05.25 | SenzaGen AB: SenzaGen's interim report January-March 2025: High activity amid global turbulence - building for growth | 202 | GlobeNewswire (Europe) | Message from the CEO"The quarter was shaped by a volatile global environment, adverse foreign exchange effects, and longer customer decision cycles, which have together impacted sales growth and consequently... ► Artikel lesen | |
| 22.04.25 | SenzaGen AB: FDA signals shift towards non-animal testing in the pharmaceutical industry - strengthens SenzaGen's commercial potential | 178 | GlobeNewswire (Europe) | The U.S. Food and Drug Administration (FDA) has announced the initiation of its planned phase-out of animal testing in the pharmaceutical industry - a decisive step towards non-animal testing methods.... ► Artikel lesen | |
| 13.02.25 | SenzaGen AB: GARD going stronger and subsidiary enters new phase | 322 | GlobeNewswire (Europe) | Message from the CEO"2024 was yet another strong year for SenzaGen with record growth for our core business GARD®, with sales increasing by 53% to nearly SEK 40 million. The Group's total revenue increased... ► Artikel lesen |
12 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| COGENT BIOSCIENCES | 37,770 | -0,47 % | Cogent Biosciences, Inc.: Cogent Biosciences Announces Multiple SUMMIT Posters at the 2026 AAAAI Annual Meeting | ||
| MINERALYS THERAPEUTICS | 31,070 | 0,00 % | Mineralys Therapeutics, Inc.: Mineralys Therapeutics' Phase 3 Launch-HTN Trial of Lorundrostat Recognized in Inaugural Journal of the American Medical Association (JAMA) "Research of the Year" Roundup | - Launch-HTN, the largest trial of an aldosterone synthase inhibitor conducted among participants with uncontrolled or treatment-resistant hypertension, was one of nine studies selected as most impactful... ► Artikel lesen | |
| CG ONCOLOGY | 53,09 | +3,94 % | Expert Outlook: CG Oncology Through The Eyes Of 7 Analysts | ||
| QIAGEN | 43,185 | +0,70 % | Qiagen übertrifft Markterwartungen im vierten Quartal | DJ Qiagen übertrifft Markterwartungen im vierten Quartal
DOW JONES--Qiagen hat im Schlussquartal 2025 den Umsatz gesteigert, der operative Gewinn ging gegenüber dem Vorjahr zurück. 2026 rechnet... ► Artikel lesen | |
| QUINCE THERAPEUTICS | 0,528 | 0,00 % | Quince Therapeutics on the brink as steroid-in-blood-cell fails in rare genetic disease | ||
| EVOMMUNE | 28,610 | 0,00 % | Evommune Starts Making Case to Challenge Dominance of Sanofi's Dupixent in Atopic Dermatitis | ||
| KYMERA THERAPEUTICS | 79,50 | 0,00 % | Kymera Therapeutics, Inc.: Kymera Therapeutics Announces First Patient Dosed in BREADTH Phase 2b Asthma Clinical Trial of KT-621, a First-in-Class, Oral STAT6 Degrader | Data from the BREADTH Phase 2b asthma trial is expected to be reported in late-2027 Data from the ongoing parallel BROADEN2 Phase 2b atopic dermatitis trial is expected to be reported by mid-2027... ► Artikel lesen | |
| ARCUTIS BIOTHERAPEUTICS | 26,880 | 0,00 % | Arcutis Biotherapeutics, Inc.: Arcutis Biotherapeutics Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| TECTONIC THERAPEUTIC | 21,050 | 0,00 % | Morning Market Movers: Abpro, Tectonic Therapeutic, Upwork, Ichor Holdings See Big Swings | AKRON (dpa-AFX) - At 6:30 a.m. ET on Tuesday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 72,88 | -0,01 % | Avidity Biosciences, Inc.: Avidity Biosciences Reports Third Quarter 2025 Financial Results and Recent Highlights | Announced Novartis agreement to acquire Avidity for total equity value of approximately $12 billion; Avidity expects to separate its early-stage precision cardiology... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 65,51 | +5,44 % | Apogee Therapeutics: Empfehlung liefert +129% in 6 Monaten | Im Juli letzten Jahres präsentierten wir im Rahmen unserer Berichterstattung und innerhalb unserer erfolgreichen Community die Aktie von Apogee Therapeutics als heißen Kauftipp. Grundlage war eine ausgeprägte... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 320,63 | 0,00 % | Guggenheim raises Praxis Precision Medicines stock price target on essential tremor opportunity | ||
| ERASCA | 11,955 | -0,46 % | Stifel bestätigt Kaufempfehlung für Erasca mit Kursziel von 10 US-Dollar | ||
| STRUCTURE THERAPEUTICS | 78,64 | -0,37 % | Structure Therapeutics Inc.: Structure Therapeutics Announces Initiation of Phase 1 Clinical Study of Oral Small Molecule Amylin Receptor Agonist ACCG-2671 for the Treatment of Obesity | SAN FRANCISCO, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Structure Therapeutics Inc. (NASDAQ: GPCR), a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for... ► Artikel lesen | |
| PHIO PHARMACEUTICALS | 1,120 | 0,00 % | Phio Pharmaceuticals Corp.: Phio Announces Safety Monitoring Committee's (SMC) Positive Wrap-up on Lead Clinical Candidate PH-762 in Skin Cancer Trial | Maximum Dose Concentration in Final Cohort Yields 85% Pathological Response (6 of 7 Patients); Complete Response (100% Tumor Clearance) in 4 of 6 RespondersNo Serious Adverse Events or Dose-Limiting... ► Artikel lesen |
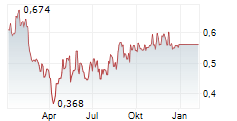
Das Unternehmen SENZAGEN AB kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 0,574 Euro und damit +2,14 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SENZAGEN AB finden Sie auf Wallstreet Online.