- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 13.02. | SenzaGen AB: SenzaGen carries out a directed issue of approximately SEK 17.5 million to the institutional investor Eiffel Investment Group | 68 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE ANNOUNCED, PUBLISHED OR DISTRIBUTED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, BELARUS, HONG KONG, JAPAN, CANADA, NEW ZEALAND, RUSSIA, SWITZERLAND... ► Artikel lesen | |
| SENZAGEN Aktie jetzt für 0€ handeln | |||||
| 13.02. | SenzaGen AB: SenzaGen's Year-End Report 2025 | SenzaGen closes the year with continued strong GARD sales | 91 | GlobeNewswire (Europe) | Message from the CEO
"As previously stated in the half-year report, 2025 performance was stronger in the second half of the year. GARD® continued to strengthen its market position with positive sales... ► Artikel lesen | |
| 16.12.25 | SenzaGen AB: SenzaGen further strengthens US market position with 1.5 MSEK order from global chemical industry leader | 122 | GlobeNewswire (Europe) | SenzaGen continues to solidify its role as an important partner for customers with high safety testing requirements, securing an assignment worth approximately 1.5 MSEK from a leading American chemical... ► Artikel lesen | |
| 20.11.25 | SenzaGen AB: SenzaGen opens expanded laboratory facilities to strengthen capacity in line with 2030 growth plan | 122 | GlobeNewswire (Europe) | To support its recently announced growth strategy through 2030, SenzaGen has opened expanded office and laboratory facilities in Lund. This investment strengthens the company's ability to meet the growing... ► Artikel lesen | |
| 18.11.25 | SenzaGen AB: UK signals shift to non-animal testing - new opportunities for SenzaGen | 142 | GlobeNewswire (Europe) | The UK government has recently unveiled a national strategy to accelerate the phase-out of animal testing in research and product development. The plan includes clear timelines and governance to ensure... ► Artikel lesen | |
| 05.11.25 | SenzaGen AB: SenzaGen's interim report January-September 2025 | Growth and break-even in Q3 | 119 | GlobeNewswire (Europe) | Message from the CEO"We delivered a strong third quarter in line with our expectations, with higher sales and significantly improved EBITDA, both sequentially and year on year, which I am pleased to... ► Artikel lesen | |
| 22.08.25 | SenzaGen AB: SenzaGen secures new follow-up order worth approx. 1.0 MSEK for GARD from a global leading U.S. pharmaceutical company | 183 | GlobeNewswire (Europe) | SenzaGen has received a new follow-up order from a global leader in the pharmaceutical and life science industry. The order, valued at approximately 1.0 million SEK, covers testing with GARD®air, SenzaGen's... ► Artikel lesen | |
| 20.08.25 | SenzaGen AB: SenzaGen's interim report January-June 2025 | Strong order intake and expanded OECD approval for GARDskin | 290 | GlobeNewswire (Europe) | Message from the CEO
"We continue to win new customers, and our order intake is significantly higher than our sales for the period, giving us a strong starting position for the coming quarters. Given... ► Artikel lesen | |
| 30.06.25 | SenzaGen AB: SenzaGen obtains expanded OECD approval for GARDskin - further strengthens position in non-animal regulatory testing | 295 | GlobeNewswire (Europe) | SenzaGen's non-animal test method GARD®skin, which has been approved since 2022 under OECD Test Guideline TG 442E, has now also been included in the newly introduced OECD TG 497. This international... ► Artikel lesen | |
| 24.06.25 | SenzaGen AB: SenzaGen receives new assignment worth 1.5 MSEK from RIFM for continued collaboration in photosensitization testing | 191 | GlobeNewswire (Europe) | SenzaGen has been awarded a new grant worth 1.5 MSEK from the Research Institute for Fragrance Materials (RIFM), a leading international research institute in fragrance safety. The assignment involves... ► Artikel lesen | |
| 14.05.25 | SenzaGen AB: Report from AGM of SenzaGen AB on 14 May 2025 | 268 | GlobeNewswire (Europe) | The Annual General Meeting (AGM) of SenzaGen held on today's date, 14 May 2025 in Lund, resolved to pass all proposals presented by the board and shareholders.
Adoption of income statement and balance... ► Artikel lesen | |
| 14.05.25 | SenzaGen AB: SenzaGen's interim report January-March 2025: High activity amid global turbulence - building for growth | 208 | GlobeNewswire (Europe) | Message from the CEO"The quarter was shaped by a volatile global environment, adverse foreign exchange effects, and longer customer decision cycles, which have together impacted sales growth and consequently... ► Artikel lesen | |
| 22.04.25 | SenzaGen AB: FDA signals shift towards non-animal testing in the pharmaceutical industry - strengthens SenzaGen's commercial potential | 186 | GlobeNewswire (Europe) | The U.S. Food and Drug Administration (FDA) has announced the initiation of its planned phase-out of animal testing in the pharmaceutical industry - a decisive step towards non-animal testing methods.... ► Artikel lesen |
13 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 40,700 | -1,44 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| BIONTECH | 86,55 | -0,23 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,304 | -6,72 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| MODERNA | 42,915 | +0,05 % | Danaher, Moderna, Emyria: Drei Biotech-Aktien für die Beobachtungsliste im März 2026 | ||
| IMMUNITYBIO | 8,566 | -0,53 % | ImmunityBio Q4 2026 Earnings Preview | ||
| BB BIOTECH | 50,20 | -3,83 % | BB Biotech: Von Innovation zu Wertrealisierung - Der Biotech-Markt in einer neuen Phase | Nach mehreren Jahren, die von erhöhten Kapitalkosten, Bewertungsdruck und ausgeprägter Risikoaversion geprägt waren, hat sich das Umfeld seit dem vergangenen Jahr spürbar stabilisiert. Finanzierungsbedingungen... ► Artikel lesen | |
| STRYKER | 330,70 | -0,12 % | Stryker highlights new additions to Mako, Triathlon portfolios at AAOS | ||
| REGENERON PHARMACEUTICALS | 663,60 | +0,58 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| VALNEVA | 4,448 | +0,41 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| AMGEN | 324,80 | +0,06 % | Amgen EVP Sold $20.77M In Company Stock | ||
| TARSUS PHARMACEUTICALS | 76,50 | -1,20 % | Tarsus Pharmaceuticals, Inc: Tarsus Pharmaceuticals Appoints Renowned Biopharmaceutical Leader and Former Allergan CEO, David Pyott, to its Board of Directors | IRVINE, Calif., Feb. 18, 2026 (GLOBE NEWSWIRE) -- Tarsus Pharmaceuticals, Inc. (NASDAQ: TARS) today announced the appointment of David E. I. Pyott, a distinguished leader in the global biopharmaceutical... ► Artikel lesen | |
| ARCUTIS BIOTHERAPEUTICS | 23,910 | -3,43 % | Arcutis Biotherapeutics, Inc.: Arcutis Begins Enrolling Phase 1a/1b Study Evaluating ARQ-234, a CD200R Agonist, in Healthy Volunteers and Adults With Atopic Dermatitis | Phase 1a/1b, first-in-human study to evaluate safety and tolerability for investigational ARQ-234 in healthy volunteers and adults with moderate to severe atopic dermatitisARQ-234 is a potent fusion-protein... ► Artikel lesen | |
| ABIVAX | 95,40 | +0,10 % | 2 Reasons Abivax Stock Could 10X by 2036 | ||
| OCUGEN | 1,568 | -1,32 % | Ocugen Announces Phase 3 liMeliGhT Enrollment Completion for OCU400, a Novel Modifier Gene Therapy for Broad Retinitis Pigmentosa | Enrollment for liMeliGhT, the first and largest gene therapy registrational trial for broad retinitis pigmentosa (RP) patients, was completed, reflecting strong interest from investigators and patientsTopline... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 49,600 | -0,40 % | Is CRISPR Therapeutics Stock Too Risky to Buy Right Now? |
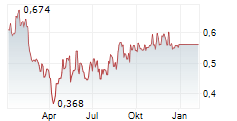
SENZAGEN AB gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,012 (+2,14 %) liegt der Kurs bei 0,574 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SENZAGEN AB finden Sie auf Wallstreet Online.