| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
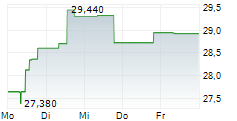
| 29,140 | 29,420 | 08:00 | |
| 29,020 | 29,280 | 08:01 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 20.02. | ALK-Abelló A/S GAAP EPS of $5.40, revenue of $6.31B | 4 | Seeking Alpha | ||
| ALK-ABELLO Aktie jetzt für 0€ handeln | |||||
| 20.02. | ALK Abello: Annual General Meeting in ALK-Abelló A/S on 16 March 2026 | 3 | GlobeNewswire (USA) | ||
| 20.02. | ALK-Abelló übertrifft Erwartungen im vierten Quartal und hebt Dividende an | 9 | Investing.com Deutsch | ||
| 20.02. | ALK Abello: Henriette Mersebach to step down as ALK's head of R&D | 110 | GlobeNewswire (Europe) | ALK (ALKB:DC / Nasdaq Copenhagen: ALK B) today announced that Henriette Mersebach will step down as Executive Vice President, Research and Development, and as a member of the Board of Management,... ► Artikel lesen | |
| 20.02. | ALK Abello: Annual report 2025: ALK delivers 15% revenue growth and 26% EBIT margin | 611 | GlobeNewswire (Europe) | ALK's (ALKB:DC / Nasdaq Copenhagen: ALK B) full-year results came in at the top end of the latest outlook, supported by solid performance in Q4 and continued commercial momentum. ALK expects sustained... ► Artikel lesen | |
| 13.02. | ALK Abello: Invitation to the presentation of ALK's annual report 2025 on Friday, 20 February 2026 | 2 | GlobeNewswire (USA) | ||
| 29.01. | ALK Abello: ALK receives positive recommendation for EURneffy 1 mg: A needle-free anaphylaxis treatment for children | 193 | GlobeNewswire (Europe) | Inside Information ALK (ALKB:DC / OMX: ALK B) today announced that the Committee for Medicinal Products for Human Use ('CHMP') of the European Medicines Agency has adopted a positive opinion recommending... ► Artikel lesen | |
| 22.12.25 | ALK Abello: ALK - Financial calendar for the 2026 financial year | 3 | GlobeNewswire (USA) | ||
| 19.12.25 | ALK Abello: Major shareholder announcement | 8 | GlobeNewswire (USA) | ||
| 01.12.25 | ALK Abello: ALK appoints Edward Jordan as new EVP and head of Commercial Operations North America | 170 | GlobeNewswire (Europe) | ALK (ALKB:DC / OMX: ALK B) today announced the appointment of Edward Jordan as new Executive Vice President (EVP) and head of Commercial Operations in North America as per 5 January 2026. Edward Jordan... ► Artikel lesen | |
| 13.11.25 | ALK-Abelló A/S reports Q3 results | 9 | Seeking Alpha | ||
| 12.11.25 | ALK-Abelló Q3 Income Rises | 3 | RTTNews | ||
| 12.11.25 | ALK Abello: Nine-month interim report (Q3) 2025 (unaudited) | 372 | GlobeNewswire (Europe) | ALK delivers 18% global organic revenue growth with operating profit up 41% in Q3 Results were better than expected, driven by a strong momentum for tablets, adrenaline autoinjectors, and SCIT/SLIT-drops.... ► Artikel lesen | |
| 12.11.25 | ALK Abello: ALK upgrades its full-year outlook | 271 | GlobeNewswire (Europe) | Inside information ALK (ALKB:DC / OMX: ALK B) today announced that the 2025 full-year financial outlook has been upgraded based on the performance in Q3 and the outlook for the remainder of the year.... ► Artikel lesen | |
| 17.09.25 | ALK Abello: ALK and GenSci partner to expand the AIT market in China | 210 | GlobeNewswire (Europe) | Inside Information GenSci is granted exclusive rights to ALK's house dust mite AIT products in China. ALK will be eligible for upfront and milestone payments of up to DKK 1.3 billion in addition to... ► Artikel lesen | |
| 08.09.25 | ALK Abello: Real-world evidence supports clinical effectiveness of the neffy nasal adrenaline spray | 169 | GlobeNewswire (Europe) | ALK (ALKB:DC / OMX: ALK B) today announced that real-world evidence evaluating the clinical performance of the nasal adrenaline spray neffy® in US patients experiencing anaphylaxis symptoms during... ► Artikel lesen | |
| 04.09.25 | ALK Abello: ALK expands the Executive Leadership Team to include key commercial regions | 2 | GlobeNewswire (USA) | ||
| 21.08.25 | ALK Abello: Six-month interim report (Q2) 2025 (unaudited) | 268 | GlobeNewswire (Europe) | ALK delivers 12% organic revenue growth with operating profit up 41% in Q2 Q2 results exceeded expectations, driven by an improved momentum for tablets and adrenaline autoinjectors. Sales in Europe... ► Artikel lesen | |
| 12.08.25 | ALK Abello: ALK upgrades its full-year revenue outlook | 273 | GlobeNewswire (Europe) | Inside information ALK (ALKB:DC / OMX: ALK B) today announced that the 2025 full-year financial outlook has been upgraded. Revenue is now expected to grow by 12-14% in local currencies (previously... ► Artikel lesen | |
| 18.07.25 | ALK Abello: EURneffy approved as the first needle-free anaphylaxis treatment of adults and children in the UK | 518 | GlobeNewswire (Europe) | Inside Information ALK (ALKB:DC / OMX: ALK B) today announced that the Medicines and Healthcare Products Regulatory Agency (MHRA) has approved EURneffy® 2 mg in the United Kingdom (UK) for anaphylaxis... ► Artikel lesen |
1 2 Weiter >>
26 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,610 | 0,00 % | Bayer verklagt Johnson & Johnson - Aktie fällt und fällt | Der DAX-Konzern Bayer will sich in den Vereinigten Staaten juristisch gegen Johnson & Johnson wehren. Der Hintergrund für die Klage ist potenziell irreführende Werbung für ein Prostatakrebs-Medikament... ► Artikel lesen | |
| NOVO NORDISK | 32,005 | -0,51 % | Hims & Hers nach Preisattacke auf Novo Nordisk mit Zahlen: Aktie rauscht erneut in den Keller | Die US-amerikanische Telemedizin-Plattform Hims & Hers hat am Montag nach Börsenschluss die Zahlen für das vierte Quartal respektive Gesamtjahr 2025 vorgelegt. Überschattet werden die Ergebnisse und... ► Artikel lesen | |
| PFIZER | 23,190 | -0,49 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 141,52 | -1,06 % | Novartis baut fünften Standort für Radioliganden-Therapie in den USA | DJ Novartis baut fünften Standort für Radioliganden-Therapie in den USA
DOW JONES--Der Schweizer Pharmakonzern Novartis hat den Bau eines weiteren Standortes für Radioligandentherapie (RLT) in... ► Artikel lesen | |
| MERCK KGAA | 126,25 | 0,00 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,00 | -0,26 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,175 | -0,47 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,31 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,930 | 0,00 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 199,20 | -0,65 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 393,15 | -0,98 % | Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament | DJ Roche erzielt klinischen Erfolg mit Multiple-Sklerose-Medikament
Von Adria Calatayud
DOW JONES--Der Schweizer Pharmakonzern Roche hat mit seinem Medikamentenkandidat gegen Multiple Sklerose... ► Artikel lesen | |
| CANOPY GROWTH | 0,929 | 0,00 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 866,40 | -0,47 % | Novo Nordisk setzt Eli Lilly unter Druck | Im frühen US-Handel zählen die Anteilscheine von Eli Lilly zu den schwächsten Titeln. Denn der dänische Rivale Novo Nordisk plant deutliche Preissenkungen für seine Blockbuster-Medikamente in den USA.... ► Artikel lesen | |
| MERCK & CO | 103,60 | -0,19 % | Leerink raises Merck stock price target on belzutifan growth |
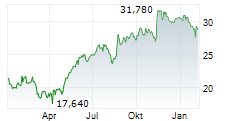
ALK-ABELLO A/S gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von +0,140 (+0,48 %) liegt der Kurs bei 29,580 Euro. Aus der Ausschüttung der letzten 12 Monate von insgesamt 0,21 Euro errechnet sich eine Dividendendrendite von +0,71 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ALK-ABELLO A/S finden Sie auf Wallstreet Online.