- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Eisai: WELIREG(R) (belzutifan) Plus LENVIMA(R) (lenvatinib) Reduced the Risk of Disease Progression or Death by 30% Compared to Cabozantinib in Certain Previously Treated Patients With Advanced Renal Cell Carcinoma (RCC) | 357 | JCN Newswire | This is the first positive Phase 3 trial of a multi-targeted tyrosine kinase inhibitor in combination with a HIF-2 alpha inhibitor, the first for patients with RCC whose disease progressed on or after... ► Artikel lesen | |
| EISAI CO LTD ADR Aktie jetzt für 0€ handeln | |||||
| Do | Eisai points the way to kidney cancer support with Kompass digital hub | 3 | FiercePharma | ||
| Do | Eisai unveils digital platform for kidney cancer patients | 4 | pharmaphorum | ||
| 19.02. | Rückschlag für Alzheimer-Mittel Lecanemab von Eisai und Biogen | 561 | dpa-AFX | BERLIN (dpa-AFX) - Für den Alzheimer-Wirkstoff Lecanemab gibt es nach Ansicht eines entscheidenden Expertengremiums keinen belegten Zusatznutzen im Vergleich zu älteren Behandlungsansätzen. Der erste... ► Artikel lesen | |
| 16.02. | Eisai: Ministry of Health, Labour and Welfare Grants Orphan Drug Designation in Japan to Novel Orexin Receptor Agonist E2086 for Narcolepsy | 455 | JCN Newswire | TOKYO, Feb 16, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") announced today that it has received an orphan drug designation for its in-house discovered and... ► Artikel lesen | |
| 10.02. | Eisai: Biologics License Application for Subcutaneous Formulation of "LEQEMBI(R)" (lecanemab) for the Treatment of Early Alzheimer's Disease Designated for Priority Review in China | 542 | JCN Newswire | TOKYO and CAMBRIDGE, Mass., Feb 10, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Biogen Inc. (Nasdaq: BIIB, Corporate headquarters: Cambridge, Massachusetts... ► Artikel lesen | |
| 09.02. | Eisai Q3 FY2025 slides reveal 109% growth in LEQEMBI sales, strategic oncology investments | 11 | Investing.com | ||
| 09.02. | Eisai GAAP EPS of ¥148.31, revenue of ¥619.95B; reaffirms FY outlook | 16 | Seeking Alpha | ||
| 06.02. | Eisai, Henlius Strike Japan Commercialization Deal for PD-1 Antibody Serplulimab | 3 | Contract Pharma | ||
| 06.02. | Leqembi starts to deliver for Eisai and Biogen | 16 | pharmaphorum | ||
| 06.02. | Eisai, Henlius partner on anti-PD-1 antibody in Japan | 2 | Investing.com | ||
| 06.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 431 | JCN Newswire | TOKYO and SHANGHAI, Feb 6, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Shanghai Henlius Biotech, Inc. (Headquarters: Shanghai, China, CEO: Jason Zhu... ► Artikel lesen | |
| 05.02. | Eisai signs $388m deal for Japanese rights to Henlius' anti-PD-1 mAb | 3 | Pharmaceutical Technology | ||
| 05.02. | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 425 | PR Newswire | TOKYO and SHANGHAI, Feb. 5, 2026 /PRNewswire/ -- Eisai Co., Ltd. ("Eisai") and Shanghai Henlius Biotech, Inc. ("Henlius") today announced the conclusion of an exclusive commercialization... ► Artikel lesen | |
| 03.02. | GHIT Fund: Total Investment of Approx. USD 8.8 Million in Malaria, Tuberculosis, and NTD R&D Projects with Partners Including Mahidol University, Barcelona Institute for Global Health, and Eisai | 1.066 | PR Newswire | TOKYO, Feb. 3, 2026 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total investment of approximately JPY 1.39 billion (USD 8.8 million1) in six R&D... ► Artikel lesen | |
| 27.01. | FDA to review Eisai's Leqembi Iqlik sBLA for Alzheimer's | 13 | Pharmaceutical Technology | ||
| 26.01. | BioArctic: Eisai submits Marketing Authorisation Variation to EMA for intravenous maintenance dosing every four weeks with Leqembi (lecanemab) | 469 | PR Newswire | STOCKHOLM, Sweden, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that they have submitted a proposed Marketing Authorisation... ► Artikel lesen | |
| 26.01. | Eisai and Biogen's subcutaneous Leqembi given FDA Priority Review for early Alzheimer's | 7 | PMLiVE | ||
| 26.01. | BioArctic's Partner Eisai Receives Priority Review For Leqembi Iqlik Subcutaneous Autoinjector | 361 | AFX News | TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics... ► Artikel lesen | |
| 26.01. | FDA grants priority review for Eisai's Alzheimer's treatment autoinjector | 8 | Investing.com |
1 2 3 4 5 Weiter >>
124 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,685 | -4,74 % | MustGrow Biologics und die Partnerschaft mit Bayer: Darum steht die Aktie erst am Anfang | Die globale Landwirtschaft steht vor einem Paradox. Sie muss mehr Menschen ernähren, aber mit weniger chemischen Mitteln. Weltweit sind in 162 Ländern inzwischen 460 Pestizide verboten oder eingeschränkt.... ► Artikel lesen | |
| NOVO NORDISK | 31,290 | -2,74 % | maydornsmeinung: Silber, Nvidia, AMD, Microsoft, IBM, SAP, TeamViewer, Novo Nordisk, Nebius & Co. | In maydornsmeinung geht es zunächst um den Gesamtmarkt: Trotz Zoll-Chaos bleiben für Alfred die Unternehmensgewinne der entscheidende Faktor. Die Berichtssaison läuft stark, auch wenn sich die großen... ► Artikel lesen | |
| PFIZER | 23,175 | -0,56 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| NOVARTIS | 140,38 | -1,86 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| GILEAD SCIENCES | 128,38 | +0,03 % | Gilead Sciences (GILD): Spannendes Chartbild - 150-USD-Trigger im Visier! | Vom HIV-Spezialisten zum Onkologie-Powerhouse! Rückblick Seit Anfang des Jahres befand sich die Aktie von Gilead Sciences in einem starken Aufwärtsimpuls, der sie von rund 122 US-Dollar bis auf ein... ► Artikel lesen | |
| AURORA CANNABIS | 3,105 | -2,66 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| GSK | 24,610 | -1,28 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 202,50 | +1,00 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 391,30 | -1,45 % | Hightech-Neubau für frühe Krankheitsdiagnosen: Drees & Sommer begleitete Roche bei Millionenprojekt | Penzberg (ots) - Im neu eingeweihten Diagnostik- und Innovationszentrum in Penzberg werden in Zukunft In-vitro-Diagnostika und spezifische Testverfahren entwickelt, um Krankheiten früh zu erkennen.Alzheimer... ► Artikel lesen | |
| CANOPY GROWTH | 0,914 | -1,61 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 869,70 | -0,09 % | Aktien New York Ausblick: Stabile Kurse vor Rede von Trump - AMD-Aktie stark | NEW YORK (dpa-AFX) - Nach dem sehr schwachen Wochenauftakt stehen die Vorzeichen für die US-Börsen am Dienstag auf Stabilisierung. Größere Sprünge nach oben sind aber nicht zu erwarten. Stattdessen... ► Artikel lesen | |
| ASTRAZENECA | 172,35 | -1,15 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,400 | -1,69 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? | ||
| TEVA | 28,200 | -2,42 % | Teva Pharmaceutical Industries Ltd: Teva to Present at the Upcoming Investor Conferences in March | PARSIPPANY, N.J. and TEL AVIV, Israel, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) today announced that Richard Francis, Teva's President and CEO, will... ► Artikel lesen |
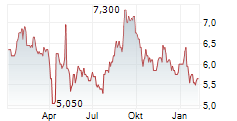
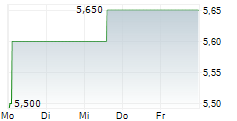
Das Unternehmen EISAI CO LTD ADR kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 6,150 Euro und damit +2,50 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EISAI CO LTD ADR finden Sie auf Wallstreet Online.