| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
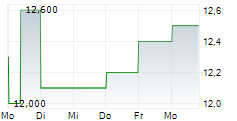
| 11,900 | 12,000 | 17:41 | |
| 11,800 | 12,000 | 17:35 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 06.02. | HUTCHMED Ltd - 6-K, Report of foreign issuer | 2 | SEC Filings | ||
| 06.02. | HUTCHMED gibt Jahresergebnisse 2025 am 5. März bekannt | 1 | Investing.com Deutsch | ||
| 06.02. | HUTCHMED to announce 2025 final results on March 5 | 1 | Investing.com | ||
| 06.02. | HUTCHMED (China) Limited: HUTCHMED to Announce 2025 Final Results | 1 | GlobeNewswire (USA) | ||
| 06.02. | HUTCHMED (00013): DATE OF BOARD MEETING AND ANNOUNCEMENT OF 2025 FINAL RESULTS | 2 | HKEx | ||
| HUTCHMED CHINA LIMITED ADR Aktie jetzt für 0€ handeln | |||||
| 06.02. | Hutchmed China Ltd - HUTCHMED to Announce 2025 Final Results | - | RNS | ||
| 14.01. | Hutchmed China hails lung cancer trial results published in Lancet | 4 | Alliance News | ||
| 14.01. | HUTCHMED Ltd - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 14.01. | Hutchmed China Ltd - Publication of Phase III Results in The Lancet | - | RNS | ||
| 14.01. | HUTCHMED Announces Publication Of Phase III SACHI Study Results In The Lancet | 388 | AFX News | LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM) on Wednesday said results from the Phase III SACHI trial evaluating savolitinib in combination with osimertinib were published in The Lancet.The... ► Artikel lesen | |
| 14.01. | HUTCHMED (China) Limited: HUTCHMED Highlights Publication of Phase III SACHI Results in The Lancet | 4 | GlobeNewswire (USA) | ||
| 14.01. | HUTCHMED (00013): VOLUNTARY ANNOUNCEMENT - HUTCHMED HIGHLIGHTS PUBLICATION OF PHASE III SACHI RESULTS IN THE LANCET | 2 | HKEx | ||
| 07.01. | Hutchmed scores with drug for rare autoimmune disease | - | pharmaphorum | ||
| 07.01. | HUTCHMED-Aktie legt zu: Sovleplenib erreicht primären Endpunkt in Phase-III-Studie | 3 | Investing.com Deutsch | ||
| 07.01. | Positive China Trial Data Moves HUTCHMED Closer To Rare Blood Disorder Drug Filing In 2026 | 1 | Benzinga.com | ||
| 07.01. | Hutchmed China hails sovleplenib results for form of anaemia in China | 1 | Alliance News | ||
| 07.01. | Hutchmed heats up autoimmune race with phase 3 win, teeing up China filing | 2 | FierceBiotech | ||
| 07.01. | Hutchmed's sovleplenib meets primary endpoint in wAIHA trial | 1 | Investing.com | ||
| 07.01. | Hutchmed China Ltd - Positive Topline Results of Phase III Trial | - | RNS | ||
| 07.01. | HUTCHMED's Sovleplenib Reaches Phase III Goal In Warm Autoimmune Hemolytic Anemia Trial In China | 364 | AFX News | LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM, HCM.L, 0013.HK) announced that the Phase III registration portion of the ESLIM-02 clinical trial evaluating sovleplenib, a novel spleen tyrosine... ► Artikel lesen |
1 2 3 4 5 Weiter >>
124 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 40,670 | -2,81 % | Märkte am Morgen: Bayer, SAP, Scout24, Knorr-Bremse, thyssenkrupp, Bitcoin | Der DAX hat eine turbulente Woche hinter sich. Am Freitag gab es noch einmal einen ordentlichen Schub nach oben. In den USA hatte der Supreme Court die Zölle von Donald Trump für unrechtmäßig erklärt.... ► Artikel lesen | |
| NOVO NORDISK | 31,790 | +0,36 % | Opening Bell: Coca-Cola, Nvidia, Eli Lilly, Novo Nordisk, Vistra Energy, Energy Fuels | An der Wall Street richtet sich die Aufmerksamkeit mal wieder auf die Zollpolitik von Donald Trump. Der Supreme Court hatte am Freitag die bisherigen Maßnahmen gekippt. Trump mit neuen Zöllen unter... ► Artikel lesen | |
| PFIZER | 23,480 | +0,38 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| NOVARTIS | 142,72 | +0,41 % | Novartis tritt in Indien den Rückzug an, um sich in Zukunft noch mehr auf das US-Geschäft konzentrieren zu können | Seit rund einem Jahr verstärkt Novartis seine Bemühungen um den US-Markt. Aus Furcht vor der trumpschen Zollkeule kündigte das Schweizer Unternehmen im vergangenen Frühjahr an, 23 Milliarden US-Dollar... ► Artikel lesen | |
| MERCK KGAA | 126,25 | +0,48 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 128,36 | +1,81 % | Gilead Sciences schlägt zu - 68 Prozent Aufschlag | Die US-amerikanische Biotech-Gesellschaft Gilead Sciences will sich im Bereich der Onkologie verstärken. Mit der geplanten Übernahme von Arcellx erwirbt das Unternehmen die volle Kontrolle über eine... ► Artikel lesen | |
| AURORA CANNABIS | 3,170 | -3,35 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| SANOFI | 81,53 | -0,26 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,900 | +0,36 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| ABBVIE | 199,60 | +1,53 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen | |
| ROCHE | 395,85 | -1,92 % | Erfolg für ROCHE | Der Schweizer Pharmakonzern hat mit seinem Medikamentenkandidat gegen Multiple Sklerose das primäre Ziel einer klinischen Studie in der Spätphase bei der häufigsten Form der Erkrankung erreicht. Die... ► Artikel lesen | |
| CANOPY GROWTH | 0,929 | -1,38 % | Is It Time to Dump Your Shares of Canopy Growth?? | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 887,40 | -0,39 % | Ausschreibung KONKRET-Preis 2026: Neues Setting, erhöhtes Preisgeld / Lilly Deutschland Stiftung intensiviert Innovationsförderung in der Gesundheitsversorgung | Bad Homburg (ots) - Mit dem Innovationspreis KONKRET fördert die Lilly Deutschland Stiftung Projekte, die Lücken und Barrieren in der Gesundheitsversorgung adressieren. Aufbauend auf bisherigen Erfolgen... ► Artikel lesen | |
| MERCK & CO | 104,20 | -0,57 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen |
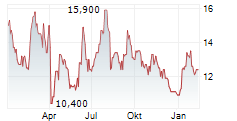
HUTCHMED CHINA LIMITED ADR gehört der Branche Pharma an. Der aktuelle Kurs liegt bei 11,400 Euro und damit -5,00 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HUTCHMED CHINA LIMITED ADR finden Sie auf Wallstreet Online.