| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,490 | 0,502 | 09:33 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 08.01. | Oncodesign Precision Medicine and Ksilink Join Forces to Test the Nanocyclix Chemical Library in Cell Models Derived From Patients With Serious Central Nervous System Diseases | 332 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Ticker symbol: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant... ► Artikel lesen | |
| 29.12.25 | Oncodesign Precision Medicine and Navigo Proteins Announce the End of Their Collaboration in the Development of Radiotheranostics | 311 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Mnemonic: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant and metastatic... ► Artikel lesen | |
| 06.11.25 | XFRA CAPITAL ADJUSTMENT INFORMATION - 06.11.2025 | 216 | Xetra Newsboard | Das Instrument ZC0 NO0012780958 STAINL.TANKERS NK 10 EQUITY wird cum Kapitalmassnahme gehandelt am 06.11.2025 und ex Kapitalmassnahme am 07.11.2025 The instrument ZC0 NO0012780958 STAINL.TANKERS NK... ► Artikel lesen | |
| 14.10.25 | Oncodesign Precision Medicine: OPM Obtains Development Grants From the Bourgogne Franche-Comté Region | 437 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Mnemonic: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant and metastatic... ► Artikel lesen | |
| 09.10.25 | Oncodesign Precision Medicine: OPM Publishes Its 2025 Half-Yearly Report | 451 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Mnemonic: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant and metastatic... ► Artikel lesen | |
| 25.09.25 | Oncodesign Precision Medicine: OPM Integrated Into the LRRK2 Investigative Therapeutics Exchange Program From the Michael J. Fox Foundation for Parkinson's Research | 352 | Business Wire | OPM will contribute its unique expertise in kinase inhibitor development and its Nanocyclix®-derived compound OPM-201 to the LITE program OPM will leverage the LITE consortium to accelerate... ► Artikel lesen | |
| ONCODESIGN PRECISION MEDICINE Aktie jetzt für 0€ handeln | |||||
| 31.07.25 | Oncodesign Precision Medicine: OPM Announces Its 2025 Half-Year Financial Results and Provides an Update on Its Clinical Developments and Financial Position | 606 | Business Wire | The REVERT Phase 1b/2a clinical trial evaluating OPM-101 in patients with advanced melanoma resistant to anti-PD1 has been submitted to the Swiss regulatory (Swissmedic) and ethics (Swiss ethics)... ► Artikel lesen | |
| 11.07.25 | Oncodesign Precision Medicine - Precision therapies for resistant diseases | 493 | Edison Investment Research | Oncodesign Precision Medicine (OPM) is a clinical-stage biopharmaceutical company listed on Euronext Growth Paris. OPM is developing precision medicine therapies targeting treatment-resistant and metastatic... ► Artikel lesen | |
| 17.04.25 | Oncodesign Precision Medicine Announces Equity Financing of up to 5 Million Euros | 422 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Mnemonic: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant and metastatic... ► Artikel lesen | |
| 03.04.25 | Oncodesign Precision Medicine: OPM Announces Its 2024 Annual Results and Clinical Developments | 596 | Business Wire | OPM-101: preclinical work establishing POC in immuno-oncology, end of phase 1 healthy volunteers and preparation for phase 1b/2a Reintegration of OPM-201 into OPM's portfolio of proprietary... ► Artikel lesen | |
| 24.03.25 | Oncodesign Precision Medicine (OPM) Announces Protocol Submission for its Phase 1b/2a Study, REVERT for OPM-101 in Combination with Pembrolizumab in Patients with Advanced Melanoma Resistant to Anti-PD1 | 747 | Business Wire | OPM-101 will be evaluated in a Phase 1b/2a clinical trial, in combination with pembrolizumab, a monoclonal antibody directed against the PD-1 protein, in patients with advanced melanoma resistant... ► Artikel lesen | |
| 04.03.25 | Oncodesign Precision Medicine: Nomination of Christophe Thurieau as an Independent Member of OPM Board of Directors | 485 | Business Wire | Regulatory News:
Oncodesign Precision Medicine (OPM) (ISIN: FR001400CM63; Mnemonic: ALOPM), a biopharmaceutical company specializing in precision medicine for the treatment of resistant and metastatic... ► Artikel lesen |
12 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| VIR BIOTECHNOLOGY | 9,340 | 0,00 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| ARCELLX | 114,05 | +0,22 % | Weekly Buzz: MGNX's LINNET Trial On Hold; Eton Pharmaceuticals, ALUR Get FDA Nod; GILD Snaps Up ACLX | FOSTER CITY (dpa-AFX) - This week, the biotech space witnessed significant milestones, including FDA approvals, oncology drug acquisitions, and collaborations. Positive clinical trial results... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 90,10 | 0,00 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| BEAM THERAPEUTICS | 28,690 | +0,74 % | Beam Therapeutics Reports Fourth Quarter and Year-End 2025 Financial Results and Announces New Liver-Targeted Genetic Disease Program in Phenylketonuria (PKU) | New Program Designed as Platform-based Approach for Direct Correction of Mutations Causing PKU; Investigational New Drug (IND) Filing for BEAM-304 Anticipated in 2026 Updated Phase 1/2 Data and Next... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,420 | -0,98 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| ARCUTIS BIOTHERAPEUTICS | 24,760 | -8,25 % | Arcutis Biotherapeutics, Inc.: Arcutis Announces Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Q4 2025 net product revenue for ZORYVE (roflumilast) was $127.5 million, an 84% increase compared to Q4 2024, and a 29% increase compared to Q3 2025Full year 2025 net product revenue for ZORYVE was... ► Artikel lesen | |
| ERASCA | 14,530 | +6,45 % | Erasca, Inc.: Erasca Announces Issuance of a U.S. Patent Covering Pan-KRAS Inhibitor ERAS-4001 | The issued patent provides intellectual property protection for ERAS-4001 and related compositions until at least 2043 Expands Erasca's diversified IP portfolio for RAS-driven cancers Initial Phase... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,630 | 0,00 % | Recursion Pharmaceuticals: Recursion Reports Fourth Quarter and Full Year 2025 Financial Results and Provides Business Update | Delivered first clinical validation of the Recursion full stack AI Operating System in FAP, demonstrating translation from AI-driven biological insight to meaningful patient outcomes; multiple clinical... ► Artikel lesen | |
| ADMA BIOLOGICS | 16,580 | 0,00 % | ADMA Biologics Announces $200 Mln Capital Return Plan | ||
| TREVI THERAPEUTICS | 12,570 | +5,54 % | Trevi Therapeutics, Inc.: Trevi Therapeutics Announces Appointment of David Hastings as Chief Financial Officer | Experienced biotech CFO to lead financial strategy and contribute to the Company's next stage of growth
NEW HAVEN, Conn., Dec. 4, 2025 /PRNewswire/ -- Trevi Therapeutics... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 70,92 | +1,26 % | RBC Capital lowers Apogee Therapeutics stock price target on dosing outlook | ||
| PRAXIS PRECISION MEDICINES | 330,19 | -1,95 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| TYRA BIOSCIENCES | 32,520 | -2,63 % | Tyra Biosciences Reports Fourth Quarter and Full-Year 2025 Financial Results and Recent Highlights | - Launched "dabogratinib 3x3" strategy to pursue 3 late-stage clinical studies in LG-UTUC, IR NMIBC and ACH, 3 potential blockbuster indications -
- Interim Ph2... ► Artikel lesen | |
| MOONLAKE IMMUNOTHERAPEUTICS | 17,680 | 0,00 % | Goldman Questions MoonLake Immunotherapeutics' (MLTX) Sonelokimab Approval Odds Despite Pipeline Update | ||
| KINIKSA PHARMACEUTICALS | 46,040 | 0,00 % | Kiniksa Pharmaceuticals International, Plc: Kiniksa Pharmaceuticals Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Portfolio Execution | - ARCALYST- (rilonacept) Q4 2025 and full year 2025 net product revenue of $202.1 million and $677.6 million, respectively -- ARCALYST 2026 net product revenue expected to be $900 - $920 million... ► Artikel lesen |
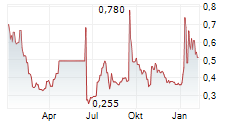
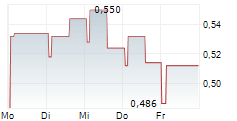
ONCODESIGN PRECISION MEDICINE SA gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -4,59 % bei einem Aktienkurs von 0,437 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ONCODESIGN PRECISION MEDICINE SA finden Sie auf Wallstreet Online.