| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,047 | 0,052 | 07.03. | |
| 0,047 | 0,052 | 06.03. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 04.02. | Hemostemix Inc (2): Hemostemix appoints Hsiang as business consultant | 3 | Stockwatch | ||
| 03.02. | Hemostemix Inc.: Hemostemix Appoints Renowned Vascular Surgeon and Principal Investigator, Dr. York Hsiang, as Business Consultant to Advance First Nations-Led Healthcare Partnerships | 171 | Newsfile | Calgary, Alberta--(Newsfile Corp. - February 3, 2026) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0), the leading autologous stem cell company treating those who suffer in pain from peripheral... ► Artikel lesen | |
| 16.01. | Hemostemix Inc.: Hemostemix Receives FDA Support for Its Basket Protocol Approach | 460 | Newsfile | Calgary, Alberta--(Newsfile Corp. - January 16, 2026) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0), the leading autologous stem cell company treating those who suffer in pain from peripheral... ► Artikel lesen | |
| 01.01. | Hemostemix Inc (2): Hemostemix closes $480,000 financing | 4 | Stockwatch | ||
| 31.12.25 | Hemostemix Inc.: Hemostemix Closes $480,000 Private Placement at $0.12 per Share | 329 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 31, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) (the "Company" or "Hemostemix"), an autologous stem cell company treating those who... ► Artikel lesen | |
| 23.12.25 | Hemostemix Inc (2): Hemostemix appoints Alper to scientific advisory board | 3 | Stockwatch | ||
| 23.12.25 | Hemostemix Inc.: Hemostemix Appoints Dr. David B. Alper, to Lead Multidisciplinary Physician Education in the Use of ACP-01 | 433 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 23, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company"), an autologous stem cell company treating those who... ► Artikel lesen | |
| HEMOSTEMIX Aktie jetzt für 0€ handeln | |||||
| 19.12.25 | Hemostemix Inc (2): Hemostemix 4,713,090-unit private placement | 4 | Stockwatch | ||
| 12.12.25 | Hemostemix Inc (2): Hemostemix grants options to buy 963,000 shares | 2 | Stockwatch | ||
| 11.12.25 | Hemostemix Inc.: Hemostemix CEO Introduces Conformal Consciousness Hypothesis A = E/(hv) | 325 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 11, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) the leading autologous (patient's own) stem cell therapy company offering VesCell (ACP-01)... ► Artikel lesen | |
| 11.12.25 | Hemostemix Inc.: Hemostemix Grants Stock Options | 205 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 11, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company") is pleased to announce that in accordance with its stock... ► Artikel lesen | |
| 11.12.25 | Hemostemix Inc (2): Hemostemix basket protocol pre-IND meeting set for Jan. | 3 | Stockwatch | ||
| 10.12.25 | Hemostemix Inc. Announces FDA Pre-IND Meeting and NBPP of $960,000 | 413 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 10, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company"), the leading autologous (patient's own) stem cell therapy... ► Artikel lesen | |
| 05.12.25 | Hemostemix Inc (2): Hemostemix appoints Harding as patient care director | 3 | Stockwatch | ||
| 04.12.25 | Hemostemix Inc.: Hemostemix Welcomes Shaune Harding, OStJ, RN as Director of Patient Care & Clinical Operations | 355 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 4, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company"), a leader in autologous stem cell therapeutics for ischemic... ► Artikel lesen | |
| 03.12.25 | Hemostemix Inc.: Hemostemix Featured at Innovations in Wound Healing 2025, Key West, December 11-14 | 442 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 3, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) ("Hemostemix" or the "Company") is pleased to announce its participation as a premier... ► Artikel lesen | |
| 02.12.25 | Hemostemix Inc.: Hemostemix to Attend DFCon San Antonio and Closed its Private Placement | 378 | Newsfile | Calgary, Alberta--(Newsfile Corp. - December 2, 2025) - Hemostemix (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0) is proud to announce its participation in DFCon 2025, hosted by the American Limb Preservation... ► Artikel lesen | |
| 27.11.25 | Hemostemix Inc (2): Hemostemix to acquire two cardiology practices | 15 | Stockwatch | ||
| 26.11.25 | Hemostemix Inc.: Hemostemix Starts its Roll-up of Cardiology Practices, Acquiring its First Two | 449 | Newsfile | Calgary, Alberta--(Newsfile Corp. - November 26, 2025) - Hemostemix Inc. (TSXV: HEM) (OTCQB: HMTXF) (FSE: 2VF0), is pleased to announce a binding Letter of Intent for the acquisition of the first two... ► Artikel lesen | |
| 21.11.25 | Hemostemix Inc (2): Hemostemix's ACP-01 dementia trial to be led by Shankle | 1 | Stockwatch |
1 2 3 4 Weiter >>
74 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| EVOTEC | 5,402 | -1,13 % | Shortseller-Positionen aktuell: Evotec, Gerresheimer, HelloFresh, Renk, Hypoport, Symrise, TUI | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| QIAGEN | 39,095 | -3,59 % | PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG | DJ PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG
Gesamtstimmrechte gemäß -- 41 WpHG
QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach... ► Artikel lesen | |
| PAION | 0,061 | -21,54 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,548 | -0,48 % | Valneva-Aktie: Die letzte Chance | Für den französischen Impfstoffentwickler Valneva entscheidet sich 2026 ein großer Teil der Investmentstory. Die Aktie wird derzeit fast ausschließlich durch die Erwartung der Phase-3-Daten zum Lyme-Borreliose-Impfstoff... ► Artikel lesen | |
| EPIGENOMICS | 0,960 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,567 | -0,22 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 314,60 | +0,16 % | William Blair reiterates Outperform on Stryker stock after analyst briefing | ||
| CRISPR THERAPEUTICS | 48,200 | -1,23 % | CRISPR Therapeutics AG (CRSP) a Moderate Buy, Per Wall Street | ||
| CORE ONE LABS | - | - | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 22.09.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 22.09.2025Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 22.09.2025ISIN NameAU000000PEK2 PEAK... ► Artikel lesen | |
| CO.DON | 0,014 | -18,18 % | EQS-AFR: CO.DON AG i.l.: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: CO.DON AG i.l.
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
CO.DON AG i.l.: Vorabbekanntmachung über die... ► Artikel lesen | |
| OCUGEN | 1,403 | +0,18 % | Ocugen Provides Business Update with Fourth Quarter and Full Year 2025 Financial Results | Enrollment for the OCU400 Phase 3 liMeliGhT clinical trial-the first and largest gene therapy registrational trial for broad retinitis pigmentosa patients-was completed. Topline Phase 3 data expected... ► Artikel lesen | |
| DAY ONE BIOPHARMACEUTICALS | 21,200 | +65,75 % | Servier; Day One Biopharmaceuticals: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs. Transaction represents... ► Artikel lesen | |
| TANGO THERAPEUTICS | 16,950 | +0,77 % | Erasca, Inc.: Erasca and Tango Therapeutics Enter into Clinical Collaboration to Evaluate Combination of ERAS-0015 and Vopimetostat | ERAS-0015, a pan-RAS molecular glue, will be evaluated in combination with PRMT5 inhibitor vopimetostat Tango will sponsor the clinical trial and Erasca will supply ERAS-0015 at no cost SAN DIEGO... ► Artikel lesen | |
| IMMUNOME | 21,040 | +0,43 % | Immunome: Q4 Earnings Insights | ||
| KINIKSA PHARMACEUTICALS | 46,160 | +0,26 % | Kiniksa Pharmaceuticals International, Plc: Kiniksa Pharmaceuticals Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Portfolio Execution | - ARCALYST- (rilonacept) Q4 2025 and full year 2025 net product revenue of $202.1 million and $677.6 million, respectively -- ARCALYST 2026 net product revenue expected to be $900 - $920 million... ► Artikel lesen |
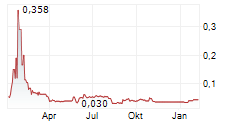
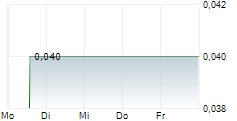
Das Unternehmen HEMOSTEMIX INC kann der Branche Biotechnologie zugeordnet werden. Der aktuelle Kurs liegt bei 0,040 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HEMOSTEMIX INC finden Sie auf Wallstreet Online.