| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,027 | 0,087 | 10:15 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | Alzinova AB: Alzinova announces final outcome of the rights issue | 95 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| Mo | Alzinova AB: Alzinova announces preliminary outcome of the rights issue | 51 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| 17.02. | Alzinova AB: Last day of trading in unit rights in Alzinovas rights issue | 154 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| 16.02. | Alzinova AB: Alzinova engages renowned international Alzheimer's expert as Global Principal Investigator ahead of planned Phase 2 study | 718 | GlobeNewswire (Europe) | Alzinova AB (publ) (Nasdaq First North: ALZ) today announces that the company has entered into a collaboration with Dr. Marwan Sabbagh, an internationally recognized Alzheimer's disease expert, who... ► Artikel lesen | |
| 06.02. | Alzinova AB: The subscription period in Alzinovas rights issue of units starts today | 170 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| 05.02. | Listing Of Units Rights And Paid Subscription Units Of Alzinova AB | 202 | GlobeNewswire | With effect from February 06, 2026, the units rights in Alzinova AB will be traded on First North Growth Market. Trading will continue up until and including February 17, 2026.
... ► Artikel lesen | |
| 03.02. | Alzinova AB: Alzinova publishes prospectus in connection with rights issue of units | 662 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| ALZINOVA Aktie jetzt für 0€ handeln | |||||
| 02.02. | XFRA CAPITAL ADJUSTMENT INFORMATION - 02.02.2026 | 500 | Xetra Newsboard | Das Instrument 78D SE0007413455 ALZINOVA AB EQUITY wird cum Kapitalmassnahme gehandelt am 02.02.2026 und ex Kapitalmassnahme am 03.02.2026 The instrument 78D SE0007413455 ALZINOVA AB EQUITY is traded... ► Artikel lesen | |
| 19.12.25 | Alzinova AB: The Board of Directors of Alzinova resolves on a rights issue of units of approximately SEK 50 million | 156 | GlobeNewswire (Europe) | NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION, IN WHOLE OR IN PART, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES, AUSTRALIA, JAPAN, NEW ZEALAND, SOUTH AFRICA, SOUTH KOREA, CANADA, THE UNITED... ► Artikel lesen | |
| 13.11.25 | Alzinova AB: Alzinova publishes interim report for January - September 2025 | 381 | GlobeNewswire (Europe) | The Board of Directors and the Chief Executive Officer of Alzinova AB hereby present the interim report for the period January - September 2025. The full report, which is attached in the press release... ► Artikel lesen | |
| 07.10.25 | Alzheimer Impfung: Alzinova: Fast Track Status von FDA erhalten - Biotech-Perle geht steil! | 853 | wallstreetONLINE | Die Aktie der schwedischen Biotech-Schmiede Alzinova schießt zu Wochenbeginn um rund 80 Prozent in die Höhe. Die Schweden haben für ihren Medikamentenkandidaten ALZ-101 von der FDA den Fast-Track Status... ► Artikel lesen | |
| 04.10.25 | Alzinova AB: Alzinova Receives Fast Track Designation from US FDA for ALZ-101 in Alzheimer's Disease | 667 | GlobeNewswire (Europe) | Alzinova AB (publ) (Nasdaq First North: ALZ) today announces that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) for the company's vaccine candidate ALZ-101 in... ► Artikel lesen | |
| 04.09.25 | Alzinova AB: Alzinova Receives FDA (IND) Approval for Phase II Study of ALZ-101 in Alzheimer's Disease | 271 | GlobeNewswire (Europe) | Alzinova AB (publ) (Nasdaq First North: ALZ) today announces that the U.S. Food and Drug Administration (FDA) has approved the company's Investigational New Drug (IND) application for its planned Phase... ► Artikel lesen | |
| 28.08.25 | Alzinova - Innovative new approach to Alzheimer's | 502 | Edison Investment Research | Alzinova is a Sweden-based clinical-stage biopharmaceutical company seeking to advance treatments for Alzheimer's disease (AD) via protein modification and immunotherapy-based solutions. Alzinova's... ► Artikel lesen | |
| 21.08.25 | Alzinova AB: Alzinova publishes half-year January - June 2025 | 299 | GlobeNewswire (Europe) | The Board of Directors and the Chief Executive Officer of Alzinova AB hereby present the half-year report for the period January - June 2025. The full report, which is attached in the press release... ► Artikel lesen | |
| 11.08.25 | Alzinova AB: Alzinova submits applications for clinical trial initiation (IND) and Fast Track status to the FDA | 408 | GlobeNewswire (Europe) | Alzinova AB (publ) (Nasdaq First North: ALZ) today announced that the company has submitted an Investigational New Drug (IND) application to the US Food and Drug Administration (FDA) for the planned... ► Artikel lesen | |
| 19.06.25 | Alzinova AB: Alzinova's ALZ-101 vaccine shows new promising results in normalizing protective antibody levels in Alzheimer's disease patients | 349 | GlobeNewswire (Europe) | Alzinova AB (ALZ) announces new clinical results showing that healthy elderly people naturally have high levels of protective antibodies against toxic amyloid-ß oligomers, which are reduced in Alzheimer's... ► Artikel lesen | |
| 17.06.25 | Alzinova AB: Alzinova selects Worldwide Clinical Trials as CRO partner for Phase 2 study of ALZ-101 | 585 | GlobeNewswire (Europe) | Alzinova AB (Nasdaq First North: ALZ) today announces a strategic collaboration with Worldwide Clinical Trials ("Worldwide"), a global leader in neuroscience studies, appointing them as the Clinical... ► Artikel lesen | |
| 28.05.25 | Alzinova AB: Bulletin from the Annual General Meeting of Alzinova AB (publ) | 279 | GlobeNewswire (Europe) | Alzinova AB (publ) (the "Company") held its Annual General Meeting ("AGM") in Gothenburg on 28 May 2025. The following resolutions were passed at the meeting.
Adoption of the annual accounts and discharge... ► Artikel lesen | |
| 15.05.25 | Alzinova AB: Alzinova publishes interim report January - March 2025 | 252 | GlobeNewswire (Europe) | The Board of Directors and the Chief Executive Officer of Alzinova AB hereby present the interim report for the period January - March 2025. The full report, which is attached in the press release,... ► Artikel lesen |
1 2 Weiter >>
25 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 93,10 | -0,21 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| EVOTEC | 5,880 | +0,38 % | Evotec Aktie: Kann man "diesem Braten wirklich trauen"? | © Foto: Julia Koblitz auf Unsplash (Symbolbild)Neue Aufträge, ein Sparprogramm und frische Analystenkommentare. Die Evotec-Aktie sendet wieder Lebenszeichen. Doch wer die Geschichte dieses Hamburger... ► Artikel lesen | |
| BB BIOTECH | 51,30 | -0,19 % | BioNTech, Valneva oder BB Biotech: Warum es HIER in Kürze knallt! | Die Biotechbranche steht zu Beginn des Jahres 2026 erneut im Spannungsfeld zwischen wissenschaftlicher Innovation und wachsender Skepsis der Kapitalmärkte. Nach Jahren extremer Kursbewegungen prüfen... ► Artikel lesen | |
| MEDIGENE | 0,046 | +14,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 41,960 | +1,38 % | Qiagen: Analysten sehen Wettbewerbssorgen als übertrieben | Nach Gesprächen mit dem Qiagen-Management auf Analysten-Konferenzen in den USA stufen Analysten die Aktie des Diagnostikspezialisten weiterhin positiv ein. Jefferies beließ es bei einer Kaufempfehlung... ► Artikel lesen | |
| MODERNA | 45,160 | -0,41 % | Moderna's combination flu, COVID shot wins over European drug regulators | ||
| VALNEVA | 4,700 | -0,63 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| BIOFRONTERA | 2,570 | -4,10 % | PTA-Adhoc: Biofrontera AG: Erhöhung Prognose | DJ PTA-Adhoc: Biofrontera AG: Erhöhung Prognose
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR Schlagwort(e): Sonstiges
Biofrontera AG: Erhöhung Prognose
Leverkusen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,990 | -1,97 % | HEIDELBERG PHARMA AG im strukturellen Gleichgewicht | ||
| BRAIN BIOTECH | 2,530 | +1,20 % | EQS-News: BRAIN Biotech AG: BRAIN Biotech verkündet 3M-Zahlen 2025/26 und Durchbruch bei BRAINBioIncubator-Beteiligung SolasCure: Aurase Wound Gel zeigt starke klinische Wirkung bei Wundreinigung und Heilung chronischer ... | EQS-News: BRAIN Biotech AG
/ Schlagwort(e): Quartals-/Zwischenmitteilung/Studienergebnisse
BRAIN Biotech verkündet 3M-Zahlen 2025/26 und Durchbruch bei BRAINBioIncubator-Beteiligung... ► Artikel lesen | |
| BIONXT SOLUTIONS | 0,386 | -1,28 % | BioNxt Solutions Inc.: BioNxt Announces Closing of Shares for Debt Settlement | VANCOUVER, BC / ACCESS Newswire / February 26, 2026 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTCQB:BNXTF)(FSE:BXT) is pleased to announce that, further to its news release dated... ► Artikel lesen | |
| ABIVAX | 101,80 | -0,97 % | EQS-News: ABIVAX: Abivax Presents First Evidence of Anti-Fibrotic Activity for Obefazimod Alongside New Clinical Efficacy and Safety Analyses in Inflammatory Bowel Disease at ECCO 2026 | EQS-News: ABIVAX
/ Key word(s): Study results
Abivax Presents First Evidence of Anti-Fibrotic Activity for Obefazimod Alongside New Clinical Efficacy and Safety Analyses in Inflammatory... ► Artikel lesen | |
| HALOZYME THERAPEUTICS | 59,08 | +0,37 % | Halozyme Therapeutics, Inc.: Halozyme Reports Full Year 2025 Record Revenue Of $1.4 Billion And Reiterates Strong 2026 Financial Guidance | Full Year 2025 Total Revenue Increased 38% YOY to Record $1.397 billionFull Year 2025 Royalty Revenue Increased 52% YOY to Record $868 million
Completed Acquisitions... ► Artikel lesen | |
| VIDAC PHARMA | 0,800 | +0,76 % | PTA-News: Vidac Pharma Holding PLC: Erster Patient in Phase-2b-Studie mit VDA-1102 bei Hochrisiko-aktinischer Keratose behandelt | DJ PTA-News: Vidac Pharma Holding PLC: Erster Patient in Phase-2b-Studie mit VDA-1102 bei Hochrisiko-aktinischer Keratose behandelt
Unternehmensmitteilung für den Kapitalmarkt
Vidac Pharma... ► Artikel lesen | |
| CARISMA THERAPEUTICS INC | 0,127 | -100,00 % | Carisma Therapeutics Inc.: Carisma Announces Delisting from Nasdaq and SEC Deregistration | PHILADELPHIA, Dec. 5, 2025 /PRNewswire/ -- Carisma Therapeutics Inc. (OTCID: CARM) (the "Company") today announced that its Board of Directors (the "Board") has... ► Artikel lesen |
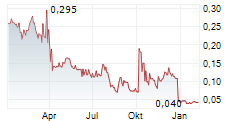
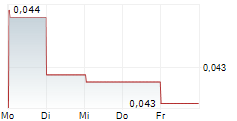
ALZINOVA AB gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 0,029 Euro und damit -3,68 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ALZINOVA AB finden Sie auf Wallstreet Online.