| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
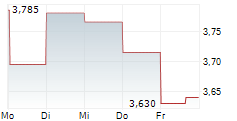
| 3,540 | 3,650 | 07.02. | |
| 3,575 | 3,705 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 272 | Business Wire | Article L233-8-II of the French Commercial Code
Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| 28.01. | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 271 | Business Wire | Article L233-8-II of the French Commercial Code
Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| 23.01. | ABIONYX Pharma Announces Its Financial Calendar for the Year 2026 | 323 | Business Wire | Regulatory News:
ABIONYX Pharma, (FR0012616852 ABNX PEA PME eligible), a next-generation biopharma company pioneering a new therapeutic era in sepsis and critical care through its proprietary... ► Artikel lesen | |
| 07.01. | ABIONYX Pharma: Half-Yearly Report on the Liquidity Contract with TP ICAP (Europe) SA | 290 | Business Wire | Regulatory News:
Under the liquidity contract covering ABIONYX Pharma shares (FR0012616852 ABNX FP) entrusted to TP ICAP (Europe), the following assets were included in the liquidity account on... ► Artikel lesen | |
| 17.12.25 | ABIONYX Pharma Carries Out a Capital Increase With Cancellation of Preferential Subscription Rights in Favor of Certain Categories of Persons, Amounting to Approximately €1.8m | 340 | Business Wire | Capital increase of approximately 1.8 million euros Subscription price: 3.10 euros Extension of financial visibility until the end of 2026
Regulatory News:
ABIONYX Pharma, (FR0012616852... ► Artikel lesen | |
| 16.12.25 | ABIONYX Pharma Is Launching a Capital Increase With Cancellation of Preferential Subscription Rights in Favor of Certain Categories of Persons, Amounting to Approximately €1.8m | 370 | Business Wire | Capital increase of approximately 1.8 million euros Subscription price: 3.10 euros Extension of visibility until the end of 2026
Regulatory News:
ABIONYX Pharma, (FR0012616852... ► Artikel lesen | |
| 05.12.25 | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 345 | Business Wire | Article L233-8-II of the French Commercial Code
Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| ABIONYX PHARMA Aktie jetzt für 0€ handeln | |||||
| 25.11.25 | ABIONYX Pharma Reports on Its Business and Cash Position for the Third Quarter of 2025 | 447 | Business Wire | Consolidated revenue of €3.06 million at the end of September 2025 Cash position of €2.8 million as of September 30, 2025
Regulatory News:
ABIONYX PharmaFR0012616852 ABNX PEA PME... ► Artikel lesen | |
| 20.11.25 | ABIONYX Pharma and SEBIA form partnership for sepsis diagnostics | 2 | Investing.com | ||
| 20.11.25 | ABIONYX Pharma and SEBIA Announce an Exclusive Global Strategic Partnership to Transform Sepsis Diagnosis | 330 | Business Wire | Regulatory News:
ABIONYX Pharma, (FR0012616852 ABNX eligible PEA PME), a next-generation biopharma company pioneering a new therapeutic era in sepsis and critical care through its proprietary... ► Artikel lesen | |
| 12.11.25 | ABIONYX Enters Strategic Discussions With IHU SEPSIS To Redefine Sepsis Treatment Landscape | 1 | RTTNews | ||
| 12.11.25 | ABIONYX Pharma Enters Advanced Strategic Discussions with IHU SEPSIS, the World's First Center Dedicated to Sepsis | 346 | Business Wire | Transformative alliance to Create the world-leading clinical infrastructure and breakthrough biotechnology to redefine the therapeutic landscape of sepsis.
ABIONYX Pharma, (FR0012616852 ABNX),... ► Artikel lesen | |
| 12.11.25 | ABIONYX Pharma Announces Advanced Strategic Discussions with IHU SEPSIS, the World's Leading Center Dedicated to Sepsis | 353 | Business Wire | A Transformative and Structuring Alliance for the Global Management of Sepsis
Regulatory News:
ABIONYX Pharma, (FR0012616852 ABNX PEA PME eligible), a next-generation biopharma company pioneering... ► Artikel lesen | |
| 04.11.25 | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 272 | Business Wire | Article L233-8-II of the French Commercial Code
Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| 21.10.25 | ABIONYX Pharma Announces Landmark Validation: A Study Published in NATURE Confirms Genetic Causality of Apolipoprotein A-I (ApoA-I) in Sepsis | 471 | Business Wire | A Global Turning Point for Critical Care Medicine
Regulatory News:
ABIONYX Pharma, (FR0012616852 ABNX PEA PME eligible), a new generation biotech company dedicated to the discovery and development... ► Artikel lesen | |
| 03.10.25 | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 393 | Business Wire | Article L233-8-II of the French Commercial Code Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma: (Paris:ABNX)
Market:... ► Artikel lesen | |
| 25.09.25 | ABIONYX Pharma: 2025 Half-Year Financial Results | 427 | Business Wire | Regulatory News:
ABIONYX Pharma, (FR0012616852 ABNX PEA PME eligible), a new generation biotech company dedicated to the discovery and development of innovative therapies based on the world's... ► Artikel lesen | |
| 05.09.25 | ABIONYX Pharma: Monthly Statement of Total Voting Rights and Shares Forming the Company's Share Capital | 388 | Business Wire | Article L233-8-II of the French Commercial Code
Article 223-16 of the General Regulations of the AMF (French Financial Markets Authority)
Regulatory News:
ABIONYX Pharma (Paris:ABNX):
Market:... ► Artikel lesen | |
| 28.08.25 | ABIONYX Pharma Provides an Update on Its Activity and Cash Position for the 2nd Quarter 2025 | 620 | Business Wire | Regulatory News:
ABIONYX Pharma (FR0012616852 ABNX PEA PME eligible), a new generation biotech company dedicated to the discovery and development of innovative therapies based on the world's... ► Artikel lesen | |
| 27.08.25 | ABIONYX Pharma Announces the Start of Discussions With a Major Player in the Field of Sepsis for a Strategic Partnership | 729 | Business Wire | Regulatory News:
ABIONYX Pharma (FR0012616852 ABNX PEA PME eligible), a new generation biotech company dedicated to the discovery and development of innovative therapies based on the world's... ► Artikel lesen |
1 2 Weiter >>
37 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | Zink-Boom, Turnaround, Biotech-Wachstum: So profitieren Sie mit Pasinex Resources, Puma und Evotec | In volatilen Märkten suchen Anleger nach außergewöhnlichen Chancen. Drei Unternehmen stechen dabei heraus. Ein Rohstoffunternehmen mit außergewöhnlichen Zink-Projekten, ein Sportartikelhersteller im... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Diagnostikkonzern Qiagen überrascht im Schlussquartal - Weiteres Wachstum 2026 | VENLO/HILDEN (dpa-AFX) - Qiagen hat 2025 von einer anziehenden Nachfrage nach seinen Kernprodukten profitiert. Dabei lief das Schlussquartal besser als vom Labordienstleister und Diagnostikkonzern... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
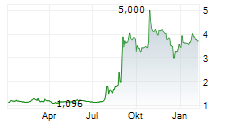
ABIONYX PHARMA SA gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,075 (-2,02 %) liegt der Kurs bei 3,640 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ABIONYX PHARMA SA finden Sie auf Wallstreet Online.