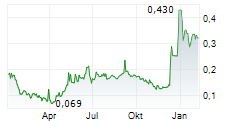
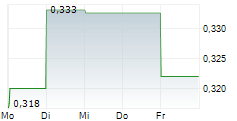
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 19.02. | Nanexa AB: Nanexa publishes year-end report and Q4 report 2025 | 80 | GlobeNewswire (Europe) | The year ended strongly with an important milestone; the license and option agreement with Moderna, which is an important validation of our technology and has created favourable conditions for 2026... ► Artikel lesen | |
| NANEXA Aktie jetzt für 0€ handeln | |||||
| 27.01. | Nanexa AB: Nanexa Announces Breakthrough Preclinical Data Demonstrating Exceptional Pharmacokinetic Profile for Monthly Semaglutide Formulation | 135 | GlobeNewswire (Europe) | Nanexa's atomic layer deposition (ALD) platform PharmaShell® demonstrably improves the pharmacokinetic profile of semaglutide injections.Significantly smoother plasma concentration curve could mitigate... ► Artikel lesen | |
| 11.12.25 | Moderna signs deal worth up to $503m with Nanexa | 37 | Pharmaceutical Technology | ||
| 11.12.25 | Moderna taps Nanexa to improve delivery of injectable therapies in back-loaded $500M pact | 19 | FierceBiotech | ||
| 10.12.25 | 59 NORTH COMMUNICATIONS: Nanexa and Moderna enter into license and option agreement for the development of PharmaShell-based products | 15 | Cision News | ||
| 10.12.25 | Nanexa AB: Nanexa and Moderna enter into license and option agreement for the development of PharmaShell-based products | 348 | GlobeNewswire (Europe) | The agreement covers the development of up to five undisclosed compounds. Nanexa will receive an upfront payment of USD 3 million and is entitled to up to USD 500 million in potential milestone payments... ► Artikel lesen | |
| 06.11.25 | Nanexa AB: Nanexa publishes interim report for January-September 2025 | 191 | GlobeNewswire (Europe) | We have progressed with the optimization of our GLP-1 formulations, extended an existing commercial partnership, received approval for a PharmaShell patent application in Japan and submitted three new... ► Artikel lesen | |
| 22.09.25 | Nanexa AB: Nanexa receives Japanese patent approval for specific PharmaShell structure | 471 | GlobeNewswire (Europe) | The approval strengthens Nanexa's patent portfolio, with additional patent applications filed in other strategically important countries
Nanexa announces that a key patent application has been granted... ► Artikel lesen | |
| 27.08.25 | Nanexa AB: Nanexa publishes interim report for January-June 2025 | 209 | GlobeNewswire (Europe) | The second quarter of 2025 has continued to validate our core belief at Nanexa that slow-release medicines can transform how we dose and therefore treat people living with a range of conditions. Reducing... ► Artikel lesen | |
| 23.05.25 | Nanexa AB: All samples analyzed from the 30 mg dose group in the NEX-22 Phase I study | 380 | GlobeNewswire (Europe) | Nanexa announced today that all pharmacokinetic (PK) samples from the final dose group, 30 mg of the Phase I study for NEX-22, now have been analyzed. The results show increased exposure in line with... ► Artikel lesen | |
| 06.05.25 | Nanexa AB: Nanexa publishes interim report for January-March 2025 | 332 | GlobeNewswire (Europe) | Very interesting data from the latest study and successful share issue.
Significant events during the first quarter of 2025In January, Nanexa announced that the company had decided to carry out a directed... ► Artikel lesen | |
| 05.05.25 | Nanexa AB: Nanexa and Applied Materials terminates collaboration | 417 | GlobeNewswire (Europe) | Nanexa and Applied Materials, Inc. have collaborated since 2020 regarding production equipment to be used for upscaling of the PharmaShell process. The companies have now agreed to terminate the collaboration.
Through... ► Artikel lesen | |
| 05.05.25 | Nanexa AB: Analysis of the first samples from the 30 mg dose group in the NEX-22 Phase I study shows continued promising results | 207 | GlobeNewswire (Europe) | Nanexa announced today that the first pharmacokinetic (PK) samples from the final dose group, 30 mg, in the ongoing Phase I study for NEX-22 have been analyzed. The results show that the PK profile... ► Artikel lesen | |
| 07.04.25 | Nanexa AB: Continued Good Tolerability of NEX-22 at 30 mg Dose of Liraglutide in the NEX-22 Study | 187 | GlobeNewswire (Europe) | Nanexa AB announces that initial observations in the phase I study show that the 30 mg dose of NEX-22, a depot formulation of liraglutide, has been well tolerated by patients with type 2 diabetes who... ► Artikel lesen | |
| 25.03.25 | Nanexa AB: Nanexa doses the last patients with 30 mg liraglutide in the NEX-22-01 study | 663 | GlobeNewswire (Europe) | Nanexa announces that all patients have now been included in the fourth and final dose cohort in the Phase I study of NEX-22, a one-month formulation of liraglutide.
NEX-22 is a new promising treatment... ► Artikel lesen | |
| 12.03.25 | Nanexa AB: Nanexa doses first patient in the 30 mg dose group in the Phase I study with NEX-22 | 327 | GlobeNewswire (Europe) | Nanexa AB announces today that the first patient has been dosed in the 30 mg dose group in the ongoing Phase I study with NEX-22, the company's one-month formulation of liraglutide.
The ongoing Phase... ► Artikel lesen |
16 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 38,155 | -6,05 % | Novo Nordisk Aktie: Schon wieder Kurssturz! - Bayer, Hometogo, Indus, Serviceware und TUI - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| NOVO NORDISK | 31,000 | -3,64 % | Lage immer dramatischer: Novo Nordisk - Aktie bricht ein. Das sind die neuen Kursziele | Die Aktie des dänischen Pharmakonzerns Novo Nordisk sorgt weiterhin für Gesprächsstoff. Die schlechten Nachrichten reißen nicht ab. Anleger sind verunsichert und genervt und werfen die Papiere aus ihren... ► Artikel lesen | |
| MERCK KGAA | 121,20 | -4,00 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| ATRIUM THERAPEUTICS | 15,060 | 0,00 % | Atrium Therapeutics Launches with Approximately $270 Million to Advance Novel RNA Medicines for Rare Genetic Cardiomyopathies | Spinoff from Novartis AG's acquisition of Avidity Biosciences advances precision cardiology programs using targeted RNA delivery platform
Lead candidates ATR 1072... ► Artikel lesen | |
| PFIZER | 22,945 | -1,54 % | Amt warnt vor Honigpaste mit verstecktem Viagra-Wirkstoff | TÜBINGEN (dpa-AFX) - Das Landratsamt Tübingen warnt vor dem Verzehr einer bei Amazon verkauften Honigpaste - weil ein verstecktes Potenzmittel drin ist. Das Nahrungsergänzungsmittel "Lotus Mixed Herbal... ► Artikel lesen | |
| SANOFI | 80,55 | -1,19 % | Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung | DJ Regeneron und Sanofi erhalten Empfehlung für erweiterte Dupixent-Zulassung
Von Colin Kellaher
DOW JONES--Der Ausschuss für Humanarzneimittel (CHMP) der Europäischen Arzneimittel-Agentur... ► Artikel lesen | |
| GSK | 24,670 | -1,04 % | GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar | DJ GSK kauft kanadische 35Pharma für 950 Millionen US-Dollar
Von Sarah Sloat
DOW JONES--GSK hat die Übernahme des Biopharma-Unternehmens 35Pharma für 950 Millionen US-Dollar vereinbart. 35Pharma... ► Artikel lesen | |
| AQUESTIVE THERAPEUTICS | 4,225 | 0,00 % | Aquestive Therapeutics, Inc. - 8-K, Current Report | ||
| GENERATE BIOMEDICINES | 12,420 | 0,00 % | Generate Biomedicines Prices IPO At $16/shr | WASHINGTON (dpa-AFX) - Generate Biomedicines, Inc. (GENB) on Thursday priced its initial public offering of 25,000,000 shares of stock at $16 per share, raising gross proceeds of $400 million.The... ► Artikel lesen | |
| ELI LILLY | 866,80 | -0,42 % | Novo Nordisk setzt Eli Lilly unter Druck | Im frühen US-Handel zählen die Anteilscheine von Eli Lilly zu den schwächsten Titeln. Denn der dänische Rivale Novo Nordisk plant deutliche Preissenkungen für seine Blockbuster-Medikamente in den USA.... ► Artikel lesen | |
| NOVARTIS | 139,04 | -2,80 % | HV-Termine: Hauptversammlungen bei BRAIN Biotech, LS Telcom, Metro, MVV Energie, Novartis | Einmal jährlich müssen sich Aufsichtsrat und Vorstand einer Gesellschaft den Aktionären stellen: Die Hauptversammlung ist das höchste Organ einer Aktiengesellschaft und vergleichbarer Unternehmens-Formen.... ► Artikel lesen | |
| DERMAPHARM | 37,600 | -5,17 % | Dermapharm: Spannend für langfristig orientierte Investoren | Am 17. März wird Dermapharm Zahlen für 2025 präsentieren. Erwartet wird von Dermapharm ein Umsatz von 1,16 Milliarden Euro bis 1,2 Milliarden Euro. Das bereinigte EBITDA sieht die Gesellschaft bei 322... ► Artikel lesen | |
| MERCK & CO | 103,40 | -0,39 % | Merck to collaborate with Tempus AI on precision oncology | ||
| BRISTOL-MYERS SQUIBB | 52,65 | -1,28 % | Bristol Myers Squibb declares $0.63 quarterly dividend | ||
| ABBVIE | 201,00 | +0,25 % | AbbVie Reports Positive Phase 3 Results For Risankizumab In Crohn's Disease | WASHINGTON (dpa-AFX) - AbbVie (ABBV) on Monday announced positive results from the Phase 3 AFFIRM study evaluating subcutaneous risankizumab in moderately to severely active Crohn's disease.... ► Artikel lesen |
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu NANEXA AB finden Sie auf Wallstreet Online.